DNA Repair

About DNA Repair
All human life originates from one single cell, the fertilised egg cell, before rapidly expanding into trillions of specialised cells which combine to form complex organ systems over a period of nine months. To maintain the delicate balance necessary for both survival and diversity, evolution has equipped us with the tools to either remove or tolerate DNA damage.
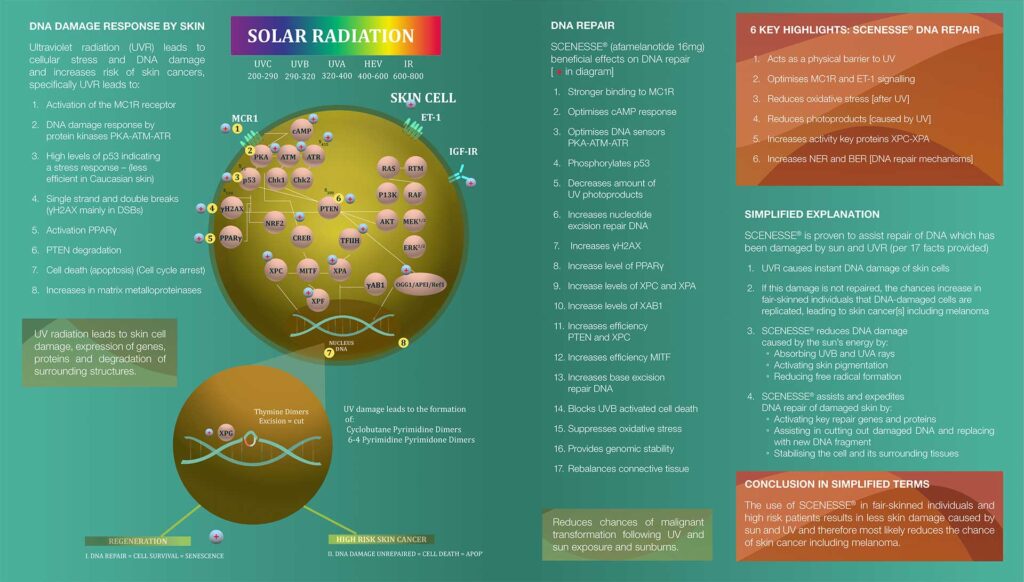
Ultraviolet radiation (UVR) in sunlight often triggers deformation of DNA within the nucleus of our skin cells. For every hour skin is exposed to the sun, approximately 80,000 of the most common forms of DNA damage occur.1 It is believed that these mutations in DNA can initiate melanoma and non-melanoma skin cancers.2
This direct damage to our DNA is accompanied by indirect damage caused by reactive oxygen species (ROS).
Our bodies have formulated a variety of strategies to repair damaged DNA, depending on the severity, and whether the cell is replicating.
- DiGiovanna, J. J., & Kraemer, K. H. (2012). Shining a light on xeroderma pigmentosum. Journal of investigative dermatology, 132(3), 785–796.
- Barnes, J. L., Zubair, M., John, K., Poirier, M. C. & Martin, F. L. Carcinogens and DNA damage. Biochemical Society Transactions 46, 1213–1224 (2018).Excision repairs, rely on identifying the damaged areas, cutting away individual or longer sections of the DNA strand and replacing them with accurate replication.
DNA damage and repair
Ultraviolet (UVB, wavelengths 290-320 nm and UVA of 320-400 nm) and high-energy visible (HEV, 380-500 nm) light penetrate the skin, leading to cellular oxidative stress and damage to DNA within the nucleus of skin cells. This damage consists of changes to the DNA structure (photoproducts) which, if left unrepaired, can replicate and increase the risk of skin cancers such as melanoma.
Under normal conditions, the body is capable of repairing DNA damage through nucleotide excision repair (NER) and base excision repair (BER); processes in which defective strands of DNA are “snipped”, removed and then replaced by the correct DNA sequences. XP patients, recipients of an organ transplant and people of Anglo-Saxon origin with red hair, blue eyes and fair skin are at the highest risk of developing skin cancers because they have either insufficient or defective NER and BER. This reduces their capacity to repair damaged DNA.
During CLINUVEL’s development of afamelanotide, a number of categories of scientific evidence have been accumulated: systemic photoprotection to skin cells; optimisation of skin cells’ response to UV radiation; anti-oxidative capacity; elimination of photoproducts (chemical damage to DNA); increased activity of DNA repair genes (as part of NER and BER); and reduction of cell death (apoptosis) following UV exposure.
Figure 1: Afamelanotide 16mg ‘DNA Repair’ – Staged R&D to date
| Stage | R&D Description |
|---|---|
| 1 | Short-term safety, repeat-dose toxicology |
| 2 | Proof of concept – human subjects
|
| 3 | Long-term safety study in clinical trials (20 years) |
| 4 | Mid-term safety commercial use (4 years) |
| 5 | Scientific evidence DNA repair
|
| 6 | Clinical evidence in
|
Figure 1 illustrates how CLINUVEL’s DNA repair programme has placed emphasis on the safety of patients and volunteers exposed to afamelanotide—more than 10,000 doses in over 1,400 subjects—during 20 years of clinical use, a requisite to being able to complete the clinical use of the hormone as a DNA restorative drug in
patients at the highest risk of developing skin cancers.
Stages S1 to S5 have been evaluated by the Company and regulatory authorities as satisfactory and complete, enabling Stage 6 of clinical investigation in the scope of afamelanotide as a DNA-regenerative pharmaceutical therapy. Clinical stage S6 consists first of a Special Access Program in XP to confirm the safety of the drug in this highest-risk population, followed by a pilot study in XP-C (CUV150), and a parallel control study in healthy volunteers (CUV151) who are exposed to UV radiation under standardised conditions.
Communiqués
SCIENTIFIC COMMUNIQUÉ X
Photoprotection and The Significance of the Minimal Erythema Dose (MED) Testing. Developing new and effective photoprotectants are paramount to curbing the ever-rising rates of skin cancer and lowering mortality rates worldwide.
View PDFSCIENTIFIC COMMUNIQUÉ IX
Beyond Pigment; The Melanocortin 1 Receptor (MC1R) in DNA Repair, details the role of the MC1R in DNA repair following light damage to our skin. It references how molecules such as afamelanotide can be used to manipulate the MC1R system and, in turn, act as one preventive strategy towards skin cancer.
VIEW PDFSCIENTIFIC COMMUNIQUÉ VIII
DNA Repair Mechanisms, answers what human DNA repair mechanisms and why are they relevant in maintaining skin health.
VIEW PDFSCIENTIFIC COMMUNIQUÉ VI
Understanding the mechanisms behind solar-radiation-induced DNA damage is a crucial step in reducing the rising incidence of skin cancer. In SCIENTIFIC COMMUNIQUÉ VI: Ultraviolet Radiation Damage and Oxidative Stress in Skin Cancer, CLINUVEL reviews the clinical significance of skin cancer as a disease and looks closely at the aetiology behind its formation.
VIEW PDFDNA Repair Communiqué I
The DNA Repair Communiqué series explores the world of the cell and provide key insights into the significance of DNA repair. This first Communiqué forms an introductory foundation, outlining fundamental aspects of cell biology and the significance of DNA repair to human survival. With this shared understanding, subsequent Communiqués will explore more detailed topics, including the role of DNA repair in oncogenesis and ageing.
VIEW PDFDNA Repair Communiqué II
The second installation in the DNA Repair Communiqué series delves deeper into the topic of DNA synthesis and repair. It establishes the fundamentals of how DNA is synthesised, focusing on the role of DNA polymerases, and reviews how cells cope with damage to the DNA template, using translesion DNA synthesis (TLS).
VIEW PDFClinical & regulatory progress
July 2024
CLINUVEL today announced the final set of results from a study (CUV151) evaluating the DNA-repair capacity of afamelanotide on skin of healthy volunteers exposed to ultraviolet (UV) radiation.
Read the announcement here.
June 2024
CLINUVEL announced the final set of results from a study evaluating the DNA-repair capacity of afamelanotide on skin of healthy volunteers exposed to UV radiation.
Read the announcement here.
February 2023
CINUVEL released the first positive results from a study evaluating the protective effects of afamelanotide on skin exposed to ultraviolet (UV) radiation (CUV151). Read the announcement here.
January 2023
CINUVEL released the first results of the Phase II study (CUV156) evaluating afamelanotide in patients with XP. Analyses showed a decrease in ultraviolet (UV) light-induced DNA skin damage following treatment. Read the announcement here.
February 2022
CLINUVEL announced that the first disease-free subjects have received afamelanotide as part of the CUV151 mechanistic study to demonstrate the drug’s potency as a DNA reparative agent. Read the announcement.
March 2022
The first xeroderma pigmentosum variant (XP-V) patient to receive afamelanotide treatment in the third active study in the Company’s DNA Repair Program (CUV152) was announced on 30 March 2022.
December 2021
CLINUVEL announced the expansion of the DNA Repair Program to the second group of xeroderma pigmentosum patients (XP-V) in the CUV156 study. Read the announcement.
October 2021
CLINUVEL announced on 5 October 2021 that it has received the necessary regulatory and ethics committee approvals to commence a new study in patients with the rare DNA repair disorder Xeroderma Pigmentosum (XP). The Phase II study (CUV156) will evaluate the safety of SCENESSE® (afamelanotide 16mg) in XP-C patients, as well as the drug’s ability to assist reparative processes following ultraviolet (UV) provoked DNA damage of the skin.
CLINUVEL announced the first XP patient treated in DNA Repair Study CUV156 on 22 October 2021.
November 2020
SCENESSE® DNA repair study in healthy volunteers (CUV151) approved. Read the announcement.
October 2020
Update released on the use of SCENESSE® (afamelanotide 16mg) in the first XP-C patient receiving treatment as part of the Company’s DNA Repair Program. Read the announcement.
September 2020
Launch of novel DNA Repair Development Program to confirm the ability of SCENESSE® (afamelanotide 16mg) to repair damaged DNA and investigate its use as a treatment for xeroderma pigmentosum (XP). Read the announcement.
References
Black JO. Xeroderma pigmentosum. Head and neck pathology. 2016 Jun;10(2):139-44.
Haddadeen C, Lai C, Cho SY, Healy E. Variants of the melanocortin‐1 receptor: do they matter clinically?. Experimental dermatology. 2015 Jan;24(1):5-9.
Green AC, Olsen CM. Increased risk of melanoma in organ transplant recipients: systematic review and meta-analysis of cohort studies. Acta dermato-venereologica. 2015 Nov 1;95(8):923-7.
Sinha RP, Häder DP. UV-induced DNA damage and repair: a review. Photochemical & Photobiological Sciences. 2002;1(4):225-36.



