CLINUVEL Expands DNA Repair Program with 2nd DNA Repair Study
| Melbourne, Australia, 14 February 2022 | ASX: XETRA-DAX: Level 1 ADR: |
CUV UR9 CLVLY |
Executive Summary:
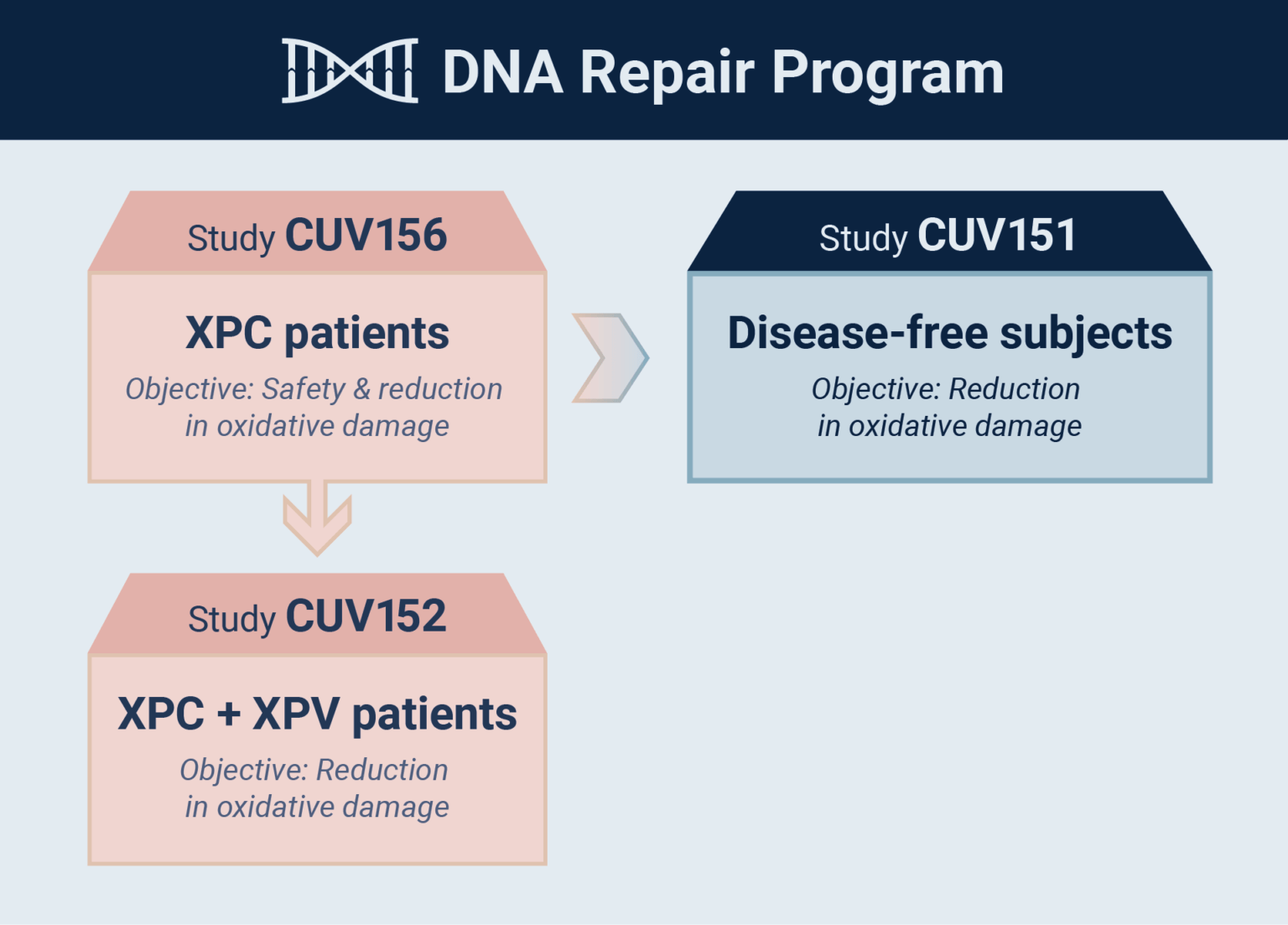
- Afamelanotide evaluated as skin DNA repair therapy in disease-free adults
- Study evaluates oxidative damage caused by UV radiation
- Focus on potency of the drug in reduction & extent of regeneration
of cellular DNA damage - Parallel study CUV156 in xeroderma pigmentosum C (XP-C) patients
CLINUVEL today announced that the first disease-free subjects have received afamelanotide as part of the CUV151 mechanistic study to evaluate the impact of the drug on DNA damaged skin. The Company ¬commenced its innovative DNA Repair Program in 2020, evaluating afamelanotide in patients with xeroderma pigmentosum (XP). The reduction of DNA damage caused by ultraviolet (UV) and solar exposure is relevant for both patients and individuals, who are at high risk of contracting skin cancer(s).
“Data from CUV151 will give us insights into afamelanotide’s ability to safely protect skin from light, and restore DNA which has incurred damage from solar exposure. The findings of this study assist us to address much broader audiences using our expertise in melanocortins and other technologies.”
“Up to two billion individuals worldwide have deficient DNA repair mechanisms of the skin,” CLINUVEL’s VP of Scientific Affairs, Dr Tim Zhou said. “Our DNA Repair Program focuses on understanding and quantifying the role of afamelanotide as an interventional therapy to help those individuals who are at greatest risk.”
Relevance of DNA Skin Damage and Repair
UV and high energy visible (HEV) light penetrate unprotected human skin, and damage DNA found in the nucleus of skin cells. If left unrepaired, DNA “photoproducts” may cause mutations, leading to skin cancer, and premature ageing (photoaging). At limited capacity, under normal conditions, our body can eliminate photoproducts through processes known as nucleotide excision repair (NER) and base excision repair (BER), where the damage is removed and replaced to restore the DNA helix.
Due to inherited genetic defects, however, many individuals have deficient DNA repair mechanisms, increasing their risk of long-term damage, and skin cancer. Particularly, XP patients belong to a group known to have the highest rate of skin cancer, due to a deficiency in the NER mechanism.
Clinically, afamelanotide has been shown to reduce photoproducts. Further research has demonstrated the ability of afamelanotide and other melanocortin molecules to assist skin cells in DNA repair mechanisms (NER and BER) as well as protecting skin from UV damage.
DNA Repair Program – CUV151 study
The mechanistic CUV151 study, conducted at an expert photodermatology unit, will expose up to ten adult disease-free subjects to afamelanotide, seeking to quantify whether the treatment can reduce DNA photoproducts and increase DNA regeneration.
“Afamelanotide 16 mg has been shown to be effective as a systemic photoprotective agent and we are now expanding the use of the molecule in patients at highest risk of skin cancers, the XP group,” CLINUVEL’s Head of Clinical Operations, Dr Pilar Bilbao said. “This current study is part of our overall DNA Repair Program to determine the mechanisms of our drug’s effects in UV-damage prone subjects, and provides a parallel protocol to the ongoing CUV156 study in XP-C patients.
“Now that disease-free subjects are allowed back in a hospital environment, the CUV151 study can proceed,” Dr Bilbao said.
– End –
About CLINUVEL PHARMACEUTICALS LIMITED
CLINUVEL (ASX: CUV; ADR LEVEL 1: CLVLY; XETRA-DAX: UR9) is a global specialty pharmaceutical group focused on developing and commercialising treatments for patients with genetic, metabolic, systemic, and life-threatening, acute disorders, as well as healthcare solutions for the general population. As pioneers in photomedicine and the family of melanocortin peptides, CLINUVEL’s research and development has led to innovative treatments for patient populations with a clinical need for systemic photoprotection, DNA repair, repigmentation and acute or life-threatening conditions who lack alternatives.
CLINUVEL’s lead therapy, SCENESSE® (afamelanotide 16mg), is approved for commercial distribution in Europe, the USA, Israel and Australia as the world’s first systemic photoprotective drug for the prevention of phototoxicity (anaphylactoid reactions and burns) in adult patients with erythropoietic protoporphyria (EPP). Headquartered in Melbourne, Australia, CLINUVEL has operations in Europe, Singapore, and the USA. For more information, please go to https://www.clinuvel.com.
SCENESSE®, PRÉNUMBRA®, and NEURACTHEL® are registered trademarks of CLINUVEL.
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD
Head of Investor Relations
Mr Malcolm Bull, CLINUVEL PHARMACEUTICALS LTD
Investor Enquiries
https://www.clinuvel.com/investors/contact-us
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance, or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products, the COVID-19 pandemic affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical products; competition for our products, especially SCENESSE® (afamelanotide 16mg); our ability to achieve expected safety and efficacy results through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, China and Japan of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE® which may lead to it being unable to supply its commercial markets and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; any failure to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2021 Annual Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on the forecasts and estimates is available on request. Past performance is not an indicator of future performance.
www.clinuvel.com
Level 11
535 Bourke Street
Melbourne – Victoria, Australia, 3000
T +61 3 9660 4900
F +61 3 9660 4999