First XP-V Patient Treated in CLINUVEL DNA Repair Study
| Melbourne, Australia, 30 March 2022 | ASX: XETRA-DAX: Level 1 ADR: |
CUV UR9 CLVLY |
Executive Summary:
- Expansion of DNA Repair Program to second group of xeroderma pigmentosum patients: XP-V
- Afamelanotide evaluated as photoprotective and DNA repair therapy
- First XP-V patient dosed
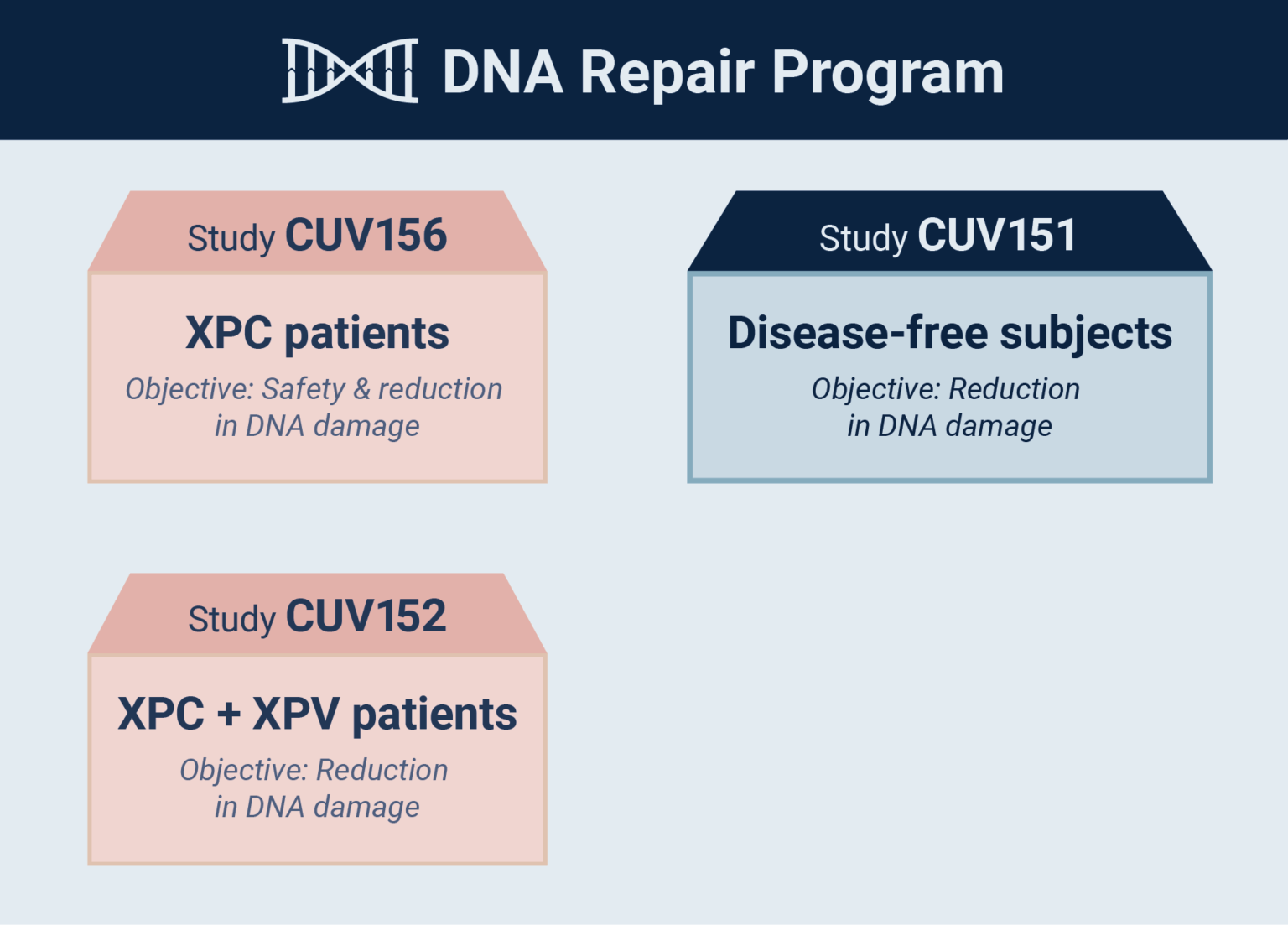
CLINUVEL today announced that the first xeroderma pigmentosum variant (XP-V) patient has received afamelanotide treatment in the third active study in the Company’s DNA Repair Program (CUV152). The CUV152 study seeks to confirm the ability of afamelanotide to protect DNA following ultraviolet- (UV) and light-induced damage.
Up to six adult patients with either the XP-C complementation group or XP-V– treated at European XP expert centres – will receive up to six doses of afamelanotide during the pilot CUV152 study.
“Deficient DNA repair mechanisms place over two billion individuals globally at increased risk of skin cancer, and XP patients are at extreme risk of solar damage and skin cancer due to their genetic defects,” CLINUVEL’s Head of Clinical Operations, Dr Pilar Bilbao said. “Our innovative DNA Repair Program is evaluating whether afamelanotide can be safely administered to these patients to both reduce and repair DNA damage, with the ultimate goal of prolonging and improving their lives.”
First systemic photoprotective treatment evaluated for XP patients
XP is a rare life-threatening inherited disorder characterised by defects in the body’s own system to repair damage due to UV light. XP-V patients’ genetic defect (POLH gene) means that as patients’ DNA is damaged following exposure to UV and light (HEV), their cells replicate with an increased number of errors, leading to an increase in mutations and overall high rate of skin cancer.
“CLINUVEL’s drug afamelanotide is the first ever systemic therapy to be evaluated in XP patients, with clinical trials CUV152 and CUV156 now focused on XP-V and XP-C, respectively,” Dr Bilbao said. “In parallel the mechanistic CUV151 study – in disease-free subjects – is providing further insights into afamelanotide’s ability to safely protect skin from light and restore DNA, which has incurred damage from light exposure.
“Depending on the centres’ ability to process the patients within the agreed timeframe, we expect to have first results from all three of these studies later in 2022. The results will help us to progress the next steps in the DNA repair program and allow discussion of late-stage development of an already marketed product with global regulatory bodies.”
Melanocortins and DNA Repair
Afamelanotide belongs to a family of bioactive peptides and their analogues – known as melanocortins – which can reduce photoproducts (pyrimidine dimers and other damage markers) caused by UV radiation and visible light. Afamelanotide protects skin from UV damage through the induction of eumelanin, the dark pigment, in skin. Eumelanin also provides antioxidative defence and has a neutralising effect on skin damage. Further research has shown the ability of melanocortins such as alpha-melanocyte stimulating hormone to assist skin cells in DNA repair mechanisms (NER and BER).¹
Having successfully commercialised afamelanotide as SCENESSE®, the world’s first photoprotective drug, CLINUVEL is developing a range of melanocortin products for use in patient populations and broader audiences.
¹ Nucleotide excision repair (NER) and Base Excision Repair (BER) are processes by which cells repair DNA damage. For further details, please see CLINUVEL’s DNA Repair Communique series.
– End –
Annex 1: Following ASX Best Practice
Name of study
A Proof of Concept, Phase IIa, Open Label Study to Evaluate the Safety and Efficacy of Subcutaneous Implants of Afamelanotide in Patients with Xeroderma Pigmentosum C and V (XPC and XPV) (CUV152).
Primary objective
- Evaluate the impact of afamelanotide on minimal erythema dose (MED) in patients with XPC and XPV
Secondary objectives
- Evaluate the impact of afamelanotide on UV-induced DNA damage and repair capacity in patients with XPC and XPV
- Evaluate the impact of afamelanotide on melanin density in patients with XPC and XPVEvaluate the safety and tolerability of afamelanotide in patients with XPC and XPV
- Evaluate the impact of afamelanotide on the skin disease severity of patients with XPC and XPV
- Evaluate the impact of afamelanotide on the quality of life of patients with XPC and XPV
Blinding status
Open label.
Product development status
Good Manufacturing Practice (GMP) Standard.
Treatment method and dose levels
Six SCENESSE® (afamelanotide 16 mg) implants
Number of trial subjects
Up to 6 XP patients.
Subject selection criteria
To be eligible to enter the study, volunteers must meet the following inclusion criteria:
- Adult males and females with genetically confirmed diagnosis of XP-C or XP-V aged between 18 and 75 years (inclusive).
- Able to understand and provide written Informed Consent prior to the performance of any study-specific procedure.
- Willing and able to comply with the conditions specified in the protocol and study procedures, in the opinion of the Investigator.
- Free of significant abnormal findings (including severe hepatic disease, hepatic impairment and renal impairment) as determined during the screening procedure by medical history and vital signs.
Further safety related inclusion and exclusion criteria apply.
Trial location
Three European XP expert clinics.
Duration of trial
Up to nine months.
Trial standard
In compliance with Good Clinical Practice (GCP) and ICH guidelines.
Annex 2: About CLINUVEL PHARMACEUTICALS LIMITED
CLINUVEL (ASX: CUV; ADR LEVEL 1: CLVLY; XETRA-DAX: UR9) is a global specialty pharmaceutical group focused on developing and commercialising treatments for patients with genetic, metabolic, systemic, and life-threatening, acute disorders, as well as healthcare solutions for the general population. As pioneers in photomedicine and the family of melanocortin peptides, CLINUVEL’s research and development has led to innovative treatments for patient populations with a clinical need for systemic photoprotection, DNA repair, repigmentation and acute or life-threatening conditions who lack alternatives.
CLINUVEL’s lead therapy, SCENESSE® (afamelanotide 16mg), is approved for commercial distribution in Europe, the USA, Israel and Australia as the world’s first systemic photoprotective drug for the prevention of phototoxicity (anaphylactoid reactions and burns) in adult patients with erythropoietic protoporphyria (EPP). Headquartered in Melbourne, Australia, CLINUVEL has operations in Europe, Singapore, and the USA. For more information, please go to https://www.clinuvel.com.
SCENESSE®, PRÉNUMBRA®, and NEURACTHEL® are registered trademarks of CLINUVEL.
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD
Head of Investor Relations
Mr Malcolm Bull, CLINUVEL PHARMACEUTICALS LTD
Investor Enquiries
https://www.clinuvel.com/investors/contact-us
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance, or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products, the COVID-19 pandemic affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical products; competition for our products, especially SCENESSE® (afamelanotide 16mg); our ability to achieve expected safety and efficacy results through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, China and Japan of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE® which may lead to it being unable to supply its commercial markets and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; any failure to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2021 Annual Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on the forecasts and estimates is available on request. Past performance is not an indicator of future performance.
www.clinuvel.com
Level 11
535 Bourke Street
Melbourne – Victoria, Australia, 3000
T +61 3 9660 4900
F +61 3 9660 4999