CLINUVEL Communiqué III
| Melbourne, Australia, 23 June 2022 |
ASX: XETRA-DAX: Level 1 ADR: |
CUV UR9 CLVLY |
Dear Shareholders, Friends
Introduction
(Mr Malcolm Bull, Head of Australian Operations & Investor Relations)
Since News Communiqué II on 31 March 2022, the Company has reported ongoing positive financial performance and progress across our expanded clinical program.
At the same time, the challenges of the operating environment have been triggered by higher-than-expected inflationary pressures in western economies, driven by higher energy prices and supply constraint remnants from the COVID-19 pandemic, exacerbated by the war in Ukraine. This has prompted monetary authorities to implement initial rounds of interest rate increases to curb the inflationary trend. These rises will likely impact economic activity until control of inflation enables renewed economic growth. The International Monetary Fund estimates world economic growth will ease from 6.1% in 2021 to 3.6% in both 2022 and 2023, with slower growth forecast in the advanced economies, while the US administration forewarns a recession. Markets have reacted adversely to inflation and higher interest rates with ‘sell-offs’ and a flight to quality. In the calendar year to the end of May 2022, the ASX / S&P 200 Index is 9.6% lower, the ASX / S&P Healthcare 200 Index is down 3.1%, and the Nasdaq Composite Index and the Nasdaq Biotechnology Index retreated by 12.1% and 21.5%, respectively. Further declines have occurred during June. The healthcare and biotech indexes encompass a wide range of companies at different stages of operation and profitability. CUV’s experience of a 41.6% fall over the year to the end of May is also the experience of many other listed small and mid-cap companies in the healthcare / biotech sector, even though CUV stands out amongst this group for being profitable and cash flow positive (for more on this, see the recent Strategic Update IV). Feedback received from discussions with global focused investors in the sector is that they are assessing their exposures, revisiting their investment thesis, and seeking reassurance in publicly traded companies with solid fundamentals. The theme heard is one of anxiety towards those biotech companies in need of continuous funding and those that are debt ridden. In this context, CLINUVEL is in a strong position with good fundamentals – a strong balance sheet and positive financial and operational performance. As markets adapt to new realities, companies in our sector with sufficient cash reserves to self-finance planned organic growth are now the preferred investment vehicles, according to some of the larger funds, hedge funds and portfolio managers.
As markets adapt to new realities, companies in our sector with sufficient cash reserves to self-finance planned organic growth are now the preferred investment vehicles, according to some of the larger funds, hedge funds and portfolio managers.
Since News Communiqué II, we have undertaken a number of meetings worldwide to reach out directly to existing and prospective shareholders face-to-face. This approach is detailed in the Communications and Investor Relations section of this News Communiqué. As you are used to from us, we continue to provide ongoing updates on the business, and specifically cover:
- an update on selected key clinical development programs, specifically:
- Stroke;
- DNA Repair; and
- Vitiligo.
- the expansion of CLINUVEL’s core competencies to non-pharmaceutical products;
- an update on US commercial operations;
- recent activities in communications and investor relations; and
- the Managing Director’s views and comments on global operations, the recent investor briefing in Basel, Switzerland, and plans for face-to-face investor events.
Clinical Programs
(Dr Pilar Bilbao, Head of Clinical Operations)
Arterial Ischaemic Stroke (AIS)
In early May, we announced the final results of the CUV801 study, a first ever study of afamelanotide in patients suffering arterial ischaemic stroke (AIS). Despite the prevalence and impact of stroke (some 15 million strokes occur worldwide annually and stroke is a leading cause of death), treatment options remain limited, particularly for patients who experience a stroke in the upper regions of the brain, where the current standards of care are unable to be deployed, or patients who arrive at a treatment centre beyond the four-and-a-half-hour therapeutic window necessary for current therapies. This unmet need clearly fits CLINUVEL’s mandates, to explore its technologies where there are no alternatives, and a strong scientific justification (backed by data) exists.
The primary focus in treating stroke patients with any new therapy must be safety, ensuring that treatment does not pose an additional burden or risk upon a patient already in an acute medical state. From this perspective, we are happy with the outcome of the CUV801 study, with no drug related adverse events (side effects) attributed to afamelanotide treatment.
It is thus of even greater comfort that the safety profile of the drug seen in CUV801 was consistent with other uses to date and allows us to look at how we may optimise the use of the drug in future studies
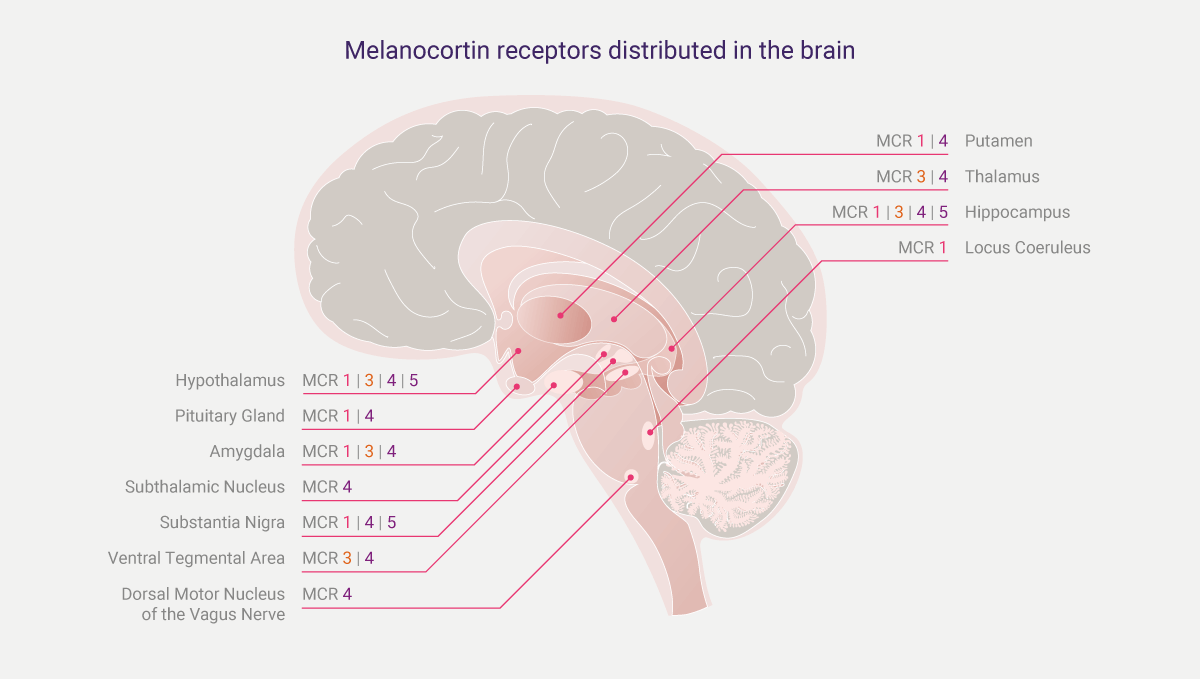
Figure 1: Melanocortin receptors distributed in the brain; these are viewed as docking stations for melanocortins to latch on and to pharmacologically activate cells.
Beyond this, there was one further, unexpected, key learning from a safety perspective. When clinical trials are established, we are deliberate in selecting the patient populations to include or exclude from study participation. While this is multifactorial – one wishes to choose a narrow enough population to be able to answer specific questions, but one which is broad enough to enable study enrolment – there are some fundamentals that are taken into account, particularly at an early phase of development. This task is, in some respects, easier in a chronic condition where patients are known to the physician, and when there are certain consistencies across the patient population (such as known medical history and potential concomitant medications).
In an acute condition such as stroke, however, you must set inclusion criteria that will allow study enrolment during a medical crisis, meaning a broader inclusion window is preferred. So it was with CUV801: in order to enrol patients, we needed to set a broader enrolment criteria. While not planned, this resulted in the majority of patients enroled in CUV801 having medical histories of cardiovascular complications, a patient population not specifically studied with afamelanotide (or melanocortins in general) and one where considerable theoretical drug-drug interactions are possible. It is thus of even greater comfort that the safety profile of the drug seen in CUV801 was consistent with other uses to date and allows us to look at how we may optimise the use of the drug in future studies.
It is thus of even greater comfort that the safety profile of the drug seen in CUV801 was consistent with other uses to date and allows us to look at how we may optimise the use of the drug in future studies.
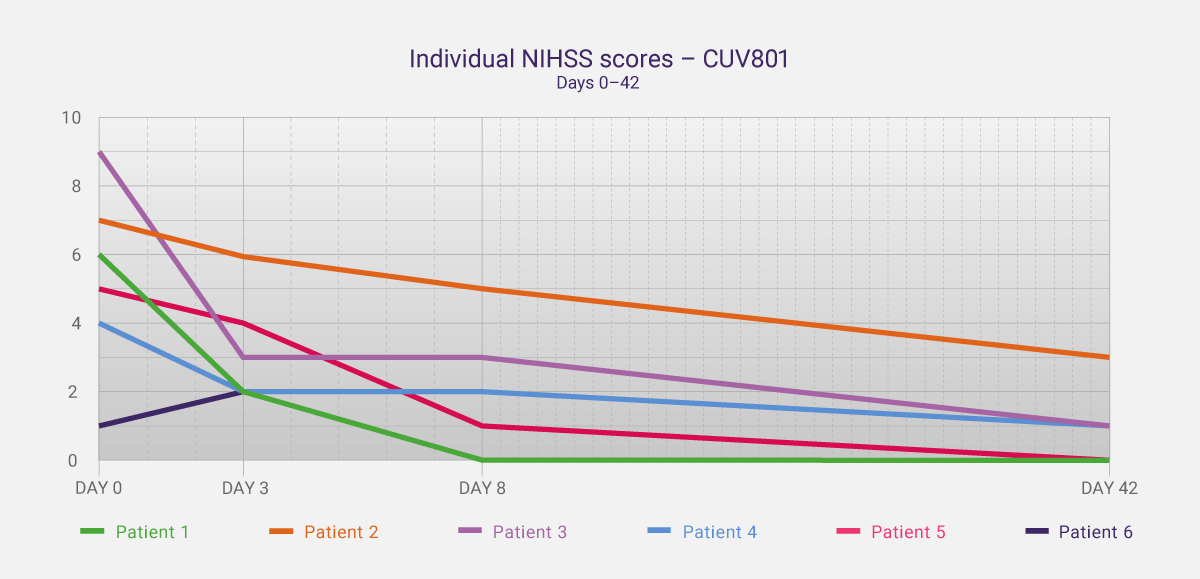
This is the exact intention of initial studies like CUV801 – to gain clinical insights and data that allow one to expand a program. In addition to the safety data, we captured key data around the effect of the treatment that are also being incorporated into further studies. The National Institutes of Health Stroke Scale (NIHSS) is a validated tool to assess a patient’s neurological function and impairment during and post-stroke (see News Communiqué II for more details). In CUV801, the NIHSS was captured up to day 42, then compared to day 0 (stroke onset). Five of the six patients in the study showed a clinically meaningful improvement in NIHSS scores (≥3 scale points), with four showing a clinically significant improvement by day 42 (≥4 scale points). Two patients were deemed to have no residual neurological impairment by day 42 (NIHSS of 0).
Clinical imaging is a fundamental to assessing stroke patients and will continue to be a central part of CLINUVEL’s program. Our interest includes the assessment of damaged brain tissue, the volume of the area affected by the stroke. Using magnetic resonance imaging (MRI-FLAIR) we can assess the extent of brain damage and recovery over time, with scans taken on days 3 and 9 of the study. Our analyses showed that five of the six patients saw a reduction in the size of the affected area over this period, albeit observations from an uncontrolled study.
Our next study, CUV803, is now advanced in planning and implementation, with enrolment set to commence in the second half of 2022. Taking these key learnings from CUV801, CUV803 will maintain a focus on safety, neurological function, and extent of damage, while evaluating a different dosing regimen to CUV801. It is clear, however, that we achieved much from this first study and the program has taken considerable steps in a short space of time.
Our next study, CUV803, is now advanced in planning and implementation, with enrolment set to commence in the second half of 2022.
DNA Repair Program
The DNA Repair Program continues apace with three studies – CUV156, CUV151 and CUV152 – continuing at expert centres. The Company’s recent Strategic Update IV outlined the full extent of the currently planned DNA Repair Program, with two further studies (CUV153 and CUV154) to be conducted pending results from the earlier program. Pending the strength of the data from the program – intending to involve 38 xeroderma pigmentosum (XP) patients and 10 disease-free control subjects – we will discuss filing a dossier from these five studies with regulatory authorities, in order to seek marketing authorisation for the product for XP.
Vitiligo
After receiving final approvals from the Institutional Review Board responsible for ethical oversight of studies at an expert centre in North America, we expect the CUV104 study to commence the recruitment of up to six patients shortly. We are excited at the prospect of evaluating afamelanotide as a monotherapy in the repigmentation of the face and body of adult vitiligo patients. Our deliberate focus is on patients with darker skin types (Fitzpatrick IV-VI), more than half a million of whom are estimated to live in Europe and North America. We have learnt how the impact of the disease is greatest for these patients and understand from our earlier studies that they may provide the greatest therapeutic response to the monotherapy treatment. I expect we will learn more from this program to be able to progress to later stage studies in 2023.
More on vitiligo can be found in Scientific Communiqués, available on the CLINUVEL website.
…we expect the CUV104 study to commence the recruitment of up to six patients shortly.

Expanding From the Core Competencies of CLINUVEL
(Dr Tim Zhao, Vice President Scientific Affairs)
The Need to Advance the Technology of Photoprotection
Society has entered the third decade of the millennium and is benefitting from the advanced technologies that significantly improve our quality of everyday life. Unfortunately, the technology of photoprotection among larger swaths of the population has not yet advanced to “twenty-first-century” level. By simply looking at the numbers of the growing skin cancer cases, it is clear that unmet needs remain high and critical, despite the availability and diversity of sun protection products. Looking on the bright side, the research and clinical experiences to date have nurtured the readiness for different photoprotection modalities and, being a world-leader in photoprotection, CLINUVEL has the unique insight, know-how, and importantly, the obligation to translate the combination of these into products to address those unmet needs.
Thanks to our years of unique experience treating erythropoietic protoporphyria (EPP) patients with SCENESSE® (afamelanotide 16mg), CLINUVEL has acquired photoprotection insight and know-how to address gaps for current photoprotection products in the market. Our approach to photoprotection is essentially a combination of:
- the polychromatic protection from the planned first over-the-counter (OTC) product;
- the utilisation of melanocortin technologies, and
- the identification of population groups with the highest need for protection.
I cannot help but get technical, so that our audiences understand the how, what, and why of our forthcoming campaigns to build new audiences. So, here we go.Having offered systemic photoprotection for years to EPP patients, CLINUVEL is offering topical (or locoregional) care for specific groups at Highest Risk of UV and high energy visible (HEV) light-induced damage. This News Communiqué provides information on our first OTC product line, which uses a number of active ingredients and chromophores to offer high polychromatic protection. Follow-on products utilising the melanocortin technology, which will offer melanogenesis, powerful antioxidative and DNA-repair enhancement effects, are the second stage.

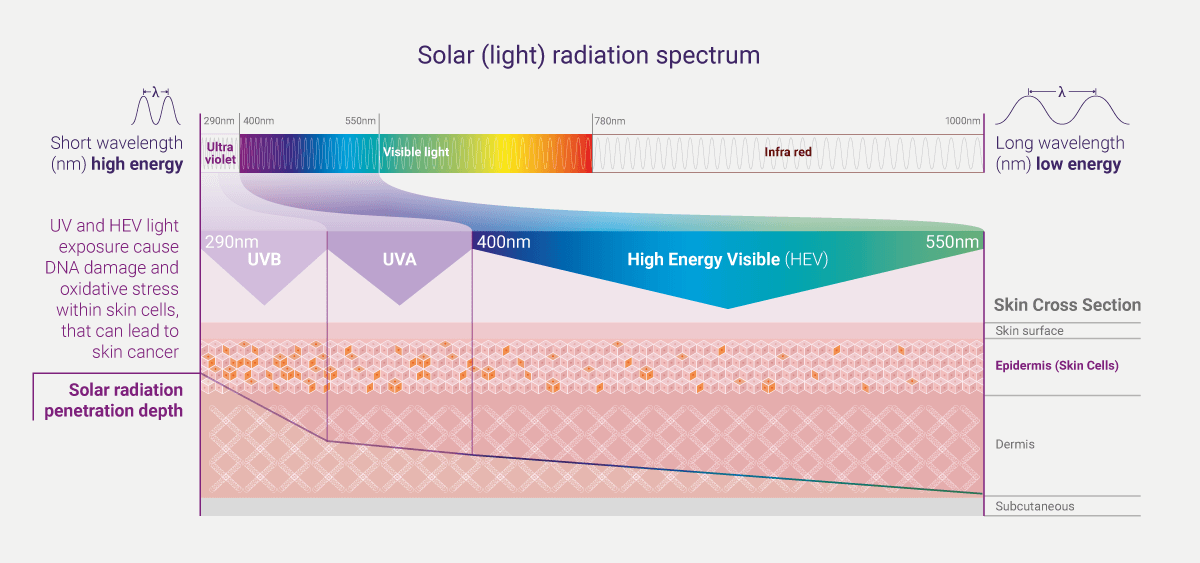
Solar exposure is the main etiological factor (cause) for all skin cancers.i Sunlight which reaches the earth is composed of 5.1% UVA, 0.3% UVB, 62.7% visible light and 31.9% infrared.ii The UV spectrum of sunlight is conventionally divided into short wavelengths (λ) UVC (λ: <190–290 nm), mid wavelengths UVB (λ: 290–320 nm), and long wavelengths UVA (λ: 320 – 400 nm). Whilst UVC is absorbed almost entirely by the earth’s ozone, solar UV wavebands do reach the surface of the earth, and as such, are of relevance for photocarcinogenesis. These include UVA and UVB, which comprise 95% and 5%, respectively, of the terrestrial sunlight UV.ii,iii Similar to UV, HEV (or blue light), can also impact skin health.
Sun and light exposure can be cytotoxic and mutagenic. UVB is absorbed directly by skin cell DNA to form DNA damage photoproducts. UVA is responsible for both the direct damage and indirect oxidative damage by the generation of reactive oxygen species (ROS), such as superoxide anion, H2O2, and singlet oxygen, which can also be induced by HEV. It is estimated that UV exposure is associated with 65% of melanoma cases and 90% of Non-melanoma skin cancer, such as basal cell carcinoma (BCC) and squamous cell carcinoma (SCC).iv The cyclobutane pyrimidine dimers (CPDs) and the 6–4 photoproducts (6-4PPs) make up around 75% and 25%, respectively, of UV-induced direct DNA photolesions.v CPDs and 6-4PPs are the major classes of mutagenic lesions that can distort the double DNA helix and interfere with transcription and replication processes, promoting base mispairing and mutagenesis.vi CPDs are believed to be the major mutagenic lesion in mammalian cells owing to their high level of induction, slow repair by the cell and replication bypass tolerance.vi Meanwhile, the generation of ROS leads to imbalance of the redox state of cells and causes damage to lipids, proteins, and DNA. Oxidative DNA damage can be formed as indirect photolesions. Guanine bases are the main target for these species. ROS-induced damage forms modified bases, apurinic/apyrimidinic sites and single strand breaks (SSBs). The addition of OH at position C8 within the guanine ring generates the oxidative product, 8-oxo-7, 8-dihydroguanine (8-oxo-dG), which is considered to be a miscoding lesion (a lesion capable of base pairing with either a cytosine or/and adenine residue) in DNA and a marker for oxidative stress.vii Oxidative DNA damage can lead to mutations and carcinogenesis.
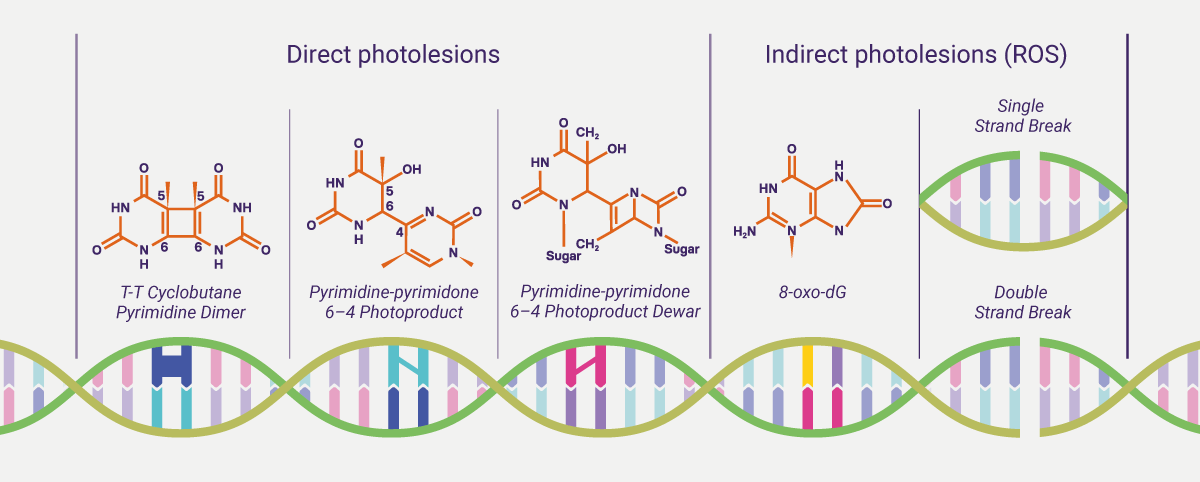
Compared to instantaneous generation of DNA lesions by UV exposure, DNA repair kinetics are relatively slow. Consequently, altered genetic information (mutation) in the presence of DNA lesions may be incorporated during DNA replication. Over time, carcinogenic mutations accumulate in skin cells, drive malignant transformation, and ultimately lead to skin cancer. Skin that is chronically sun-exposed over the long-term accumulates a large number of mutations, approaching the mutation frequency present in cancers of internal organs not exposed to light. The gradual acquisition of cancer-related mutations that drive malignant transformation leads to skin cancer, and DNA lesions generated from UV radiation most commonly lead to C>T mutations at dipyrimidine sites, which are termed as the UV signature mutation.viii
Our skin makes up 16% of our total body weight and is one of our largest organs.ix Its ability to prevent or remove DNA damage is critical for prevention of the malignant transformation of healthy skin cells into cancer. Human skin utilises multiple approaches to achieve this, including activating DNA repair mechanisms, apoptosis, antioxidative enzyme upregulation, as well as production of eumelanin, which is a physical barrier that reduces the penetration of UV and visible light through the epidermis. While such photoprotection capabilities of the human skin are efficient in cancer prevention, they are not always sufficient, as evidenced by the high incidence of skin cancer cases. Notably, higher risks of photodamage and carcinogenesis can be experienced by people with reduced photoprotection capability, or by people with increased photo-exposure.
As an example, patients with XP have at least a 1,000-fold higher risk for the deadliest skin cancer, melanoma, than the general population due to defects in their DNA repair capability.x People with very fair – that is Caucasian – skin (Fitzpatrick I-II) are also at higher risk of skin cancer. Their reduced ability to produce pigmentation (melanogenesis), which is mainly regulated by the melanocortin 1 receptor (MC1R), leads to a reduced defence capability. Others are at higher risk again. Patients who have received an organ transplant are at considerably higher risk of SCC (100 times) and BCC (six times) compared to the general population. Similarly, people with a family history of skin cancer may have less ability to repair DNA damage, a reduced ability to remove the mutated cells, or a higher chance of pro-cancerous mutations caused by photo-induced DNA damage. Outside of these groups, individuals who chronically ‘over-expose’ to solar light may overwhelm their skin’s photoprotection capability, which can lead to damage accumulation and increase the chance of mutation in the genome that leads to skin cancer.
For the three vulnerable populations stated above, their protective systems in the skin are either operating at a reduced competence or at an insufficient state, meaning they are unable to handle the continuous and long-term effects of sun damage, placing them at much higher risk of skin cancer. One solution is to reduce the total light exposure with high protection-factor polychromatic screen covering both UV and HEV. Another is to enhance these protective systems through pharmacological manipulation. These subjects are the core knowledge and business of CLINUVEL.
References and relevant research
- Hearing V.J., et al., (2005) Mutation Res. 571, 133.
- Chatterjee N., et al. (2017) Mutagen. 58, 235.
- Pfeifer G. P., et al., (2012) Photochem. Photobiol. Sci. 11, 90.
- Kim I., et al. (2014) Genes & Diseases, 1, 188.
- Sinha R. P., et al., (2002) Photochem. Photobiol. Sci. 1, 225.
- Jarrett S. G., et al., (2017) Pigment Cell Melanoma Res. 30, 284
- Barnes J. L., et al., (2018) Biochemical Society Transactions 46, 1213.
- Lee J. W., et al., (2020) Photochem Photobiol. 96, 478.
- Leider M., (1949) J Invest Dermatol. 12, 187.
- Kraemer K. H., et al., (1994) Arch. Derm. 130, 1018.Many of the centres involved in these publications have worked or engaged with CLINUVEL’s R&D over the past two decades.
Developing Healthcare Solutions
For over a decade, through our lead pharmaceutical product SCENESSE®, CLINUVEL has been offering total body photoprotection to one of the most extreme photoprotection-incompetent populations, EPP patients, who are characterised by their minimum tolerance to blue light. SCENESSE®, the world’s first and only approved product for systemic photoprotection, has demonstrated proven and promising applications of the melanocortin technologies to allow EPP patients to lead more normal lives. With this experience, CLINUVEL has the know-how to protect those at greatest risk from UV and HEV.
The first OTC product line from our Healthcare Solutions Division will be complementing SCENESSE® in many ways, targeting our patients in dermatology practices and clinical trials and specific ‘Highest Risk’ populations:
- three vulnerable populations;
- from prescription to over-the-counter: products will be made directly available for end user supply;
- from injectable drug to topical semi-solid application;
- from systemic protection to locoregional protection: in-need consumers will decide when to apply for required protection.
The first OTCs offer polychromatic protection to provide wider-spectrum protection over different wavelengths, more advanced than existing solar protection products. In addition to UVB and UVA protection by a broad-spectrum sunscreen, CLINUVEL’s Polychromatic Screen also provides effective protection against HEV light, which is the wavelength band responsible not only for phototoxicity in EPP patients, but also for the higher oxidative stress in the human skin in general. As a second step, and as second product line, our new generation melanocortin-based products are on the way to serve non-prescription markets. Originated from a highly regulated environment, those translated non-prescription products will offer differentiating and genuine care to users, by enhancing all the major pathways that our skin utilises to prevent skin cancers early in the process:
- enhance melanogenesis to reduce photo exposure to both UV and HEV;
- induce antioxidative enzymes activities to reduce oxidative stress; and
- enhance DNA repair to help reduce or prevent mutation and carcinogenesis.
While focusing and dedicating our resources on product development, technology, and innovation, CLINUVEL is releasing communication and educational campaigns to reach the identified populations at Highest Risk to raise global awareness. With the scientific expertise and clinical know-how established over years of devotion, CLINUVEL’s communications and marketing serves to address several important areas. These include providing information on specific physical and behavioral risks, needs and prospective solutions for the at-risk populations.
The first OTCs offer polychromatic protection to provide wider-spectrum protection over different wavelengths, more advanced than existing solar protection products.
Establishing Global Communities First
CLINUVEL’s strategy is to address solar challenge globally: photoprotection at the very early stage to prevent skin cancer development. Early intervention will include awareness, a long-term view and accurate information, while converting to polychromatic screens, and in a second stage to melanocortins to reduce photo-induced DNA damage, with emphasis on Highest Risk populations.
By establishing global communities associated with and dedicated to the challenge of changing a worldwide epidemic of skin cancer, CLINUVEL is deploying an extensive digital marketing strategy. This will utilise the knowledge ambassadors from highest Risk Communities have, engaging with our target audiences to share their own experiences of photodamage and photoprotection alongside educational content. This will be supported by the creation of digital spaces, across multiple platforms, for those at Highest Risk of solar damage to engage with both CLINUVEL and each other, fostering a community dedicated to photoprotection and skin cancer prevention.

Testing and Developing the Market with the First OTCs
The launch of CLINUVEL’s very first non-prescription product, as with any first product launch, will be a test of the newly formed audiences. Key to this is the delivery of our educational and awareness campaigns, sharing knowledge on technologies, physics phenomenon, optics, and skin care, summarising what we have learned about sunlight interacting with our skin. The effects of solar exposure impact those of us who suffer the greatest consequences.
To assist sharing this knowledge will be our trusted CLINUVEL Ambassadors (CUVAs), joining us from each of the groups in our community who are at the very-high states of need (albeit, sometimes representatives do not even realise the threat themselves). The aim is for them to speak a language just like their peers and the broader general population.
To assist sharing this knowledge will be our trusted CLINUVEL Ambassadors (CUVAs), joining us from each of the groups in our community who are at the very-high states of need
The past week, the first CUVAs went live. Our dedicated platform is LightSkinScience.com. We invite you to follow CLINUVEL’s expanding online campaigns.
US Operations
(Dr Linda Teng, Director of North American Operations)
Since the beginning of this year, the US team has been assisting the EPP Specialty Centers and EPP patients in renewing their health insurance’s prior authorization (PA) to ensure continuous treatment access. Some insurance companies require patients to renew the PA every 6-12 months, while some health insurers do not require PA for coverage of the SCENESSE® medication.
The US team is fortunate to work with a select group of US EPP Specialty physicians who are passionate about treating EPP patients. Many of these Centers have incorporated EPP into their residency and fellowship programs, imparting knowledge to the next generation of healthcare providers. Their enthusiasm and dedication toward their EPP patients shine through and are greatly acknowledged and appreciated by their EPP patients. The US team continues to identify new EPP Specialty Centers, having trained and accredited additional EPP Centers early this year, with more scheduled in the coming months. The EPP Specialty Centers enable patients to receive better healthcare and treatment closer to their homes.
The geographical location of the EPP patients often dictates the frequency of EPP treatment. EPP patients residing in the southern region of the US receive regular treatments all year-round while EPP patients residing in the northern region of the US receive treatment mainly during the spring and summer seasons when they are at highest risk from light exposure.
The US team have become acquainted with the EPP patients as many are entering their third year of treatment. We have received positive feedback and the team looks forward to assisting new US EPP patients in the coming months.
The US team had become acquainted with the EPP patients as many are entering their third year of treatment.
Communications and Investor Relations
(Mr Malcolm Bull, Head of Australian Operations & Investor Relations)
Recent Announcements
The Company’s recent announcements are listed below:
| Date | Announcement |
|---|---|
| 01 April | CLINUVEL Extends CEO Employment Agreement |
| 04 April | CLINUVEL Appoints New Non-Executive Director |
| 06 April | Chair’s Letter |
| 07 April | Initial Director’s Interest Notice |
| 27 April | Notice of Ceasing to be a Substantial Holder |
| 28 April | Quarterly Activities / Appendix 4C Cash Flow Report |
| 29 April | CEO Letter |
| 04 May | Positive Final Results in Stroke Study CUV801 |
| 06 May | Notification Regarding Unquoted Securities |
| 10 May | CLINUVEL Progresses CUV104 Vitiligo Study |
| 12 May | Change of Director’s Interest Notice x2 |
| 13 May | CLINUVEL Strategic Update IV |
| 20 May | Becoming a Substantial Shareholder |
| 25 May | Change in Substantial Holding |
| 26 May | H. C. Wainwright Global Investment Conference Presentation |
| 14 Jun | Ceasing to be a Substantial Shareholder |
| 14 Jun | Change of Director’s Interest Notice |
| 23 Jun | News Communiqué III |
All of CLINUVEL’s announcements are available on the CLINUVEL website and CLINUVELNews. More specifically, announcements to the Australian Securities Exchange are available on the investor pages of the CLINUVEL website.
Pace of Communications
We have increased the number and diversity of communications in the calendar year to 23 June 2022. Thirty-six announcements have been made to the Australian Securities Exchange compared to 23 in the same period of 2021. In addition, we have distributed an investor webinar and a scientific communiqué to stakeholders in the year to date. The feedback received from the financial community on the quality of these communications has been positive and encouraging.
Recap of Financial Results
Since News Communiqué II, we announced the cash receipts and activity report for the March Quarter 2022. Increased efficiencies in product distribution, more prescribing centres, and rising patient demand for SCENESSE® were the main factors contributing to positive receipts of A$11.442 million for the March quarter for the Group. The further strength in performance enabled the Company to reinvest in research and development and attract additional staff talent. In parallel, and according to projections, expenditures remained higher than average across the business units to provide for long-term growth. Overall, the Group recorded – for the first-time – cash and cash equivalents exceeding A$100 million, serving as a buffer to respond to unforeseen adverse events and enabling further investments.
Cash receipts have been a record for a quarter in each of the September, December, and March quarters to date of the 2022 financial year.
Cash receipts have been a record for a quarter in each of the September, December, and March quarters to date of the 2022 financial year.
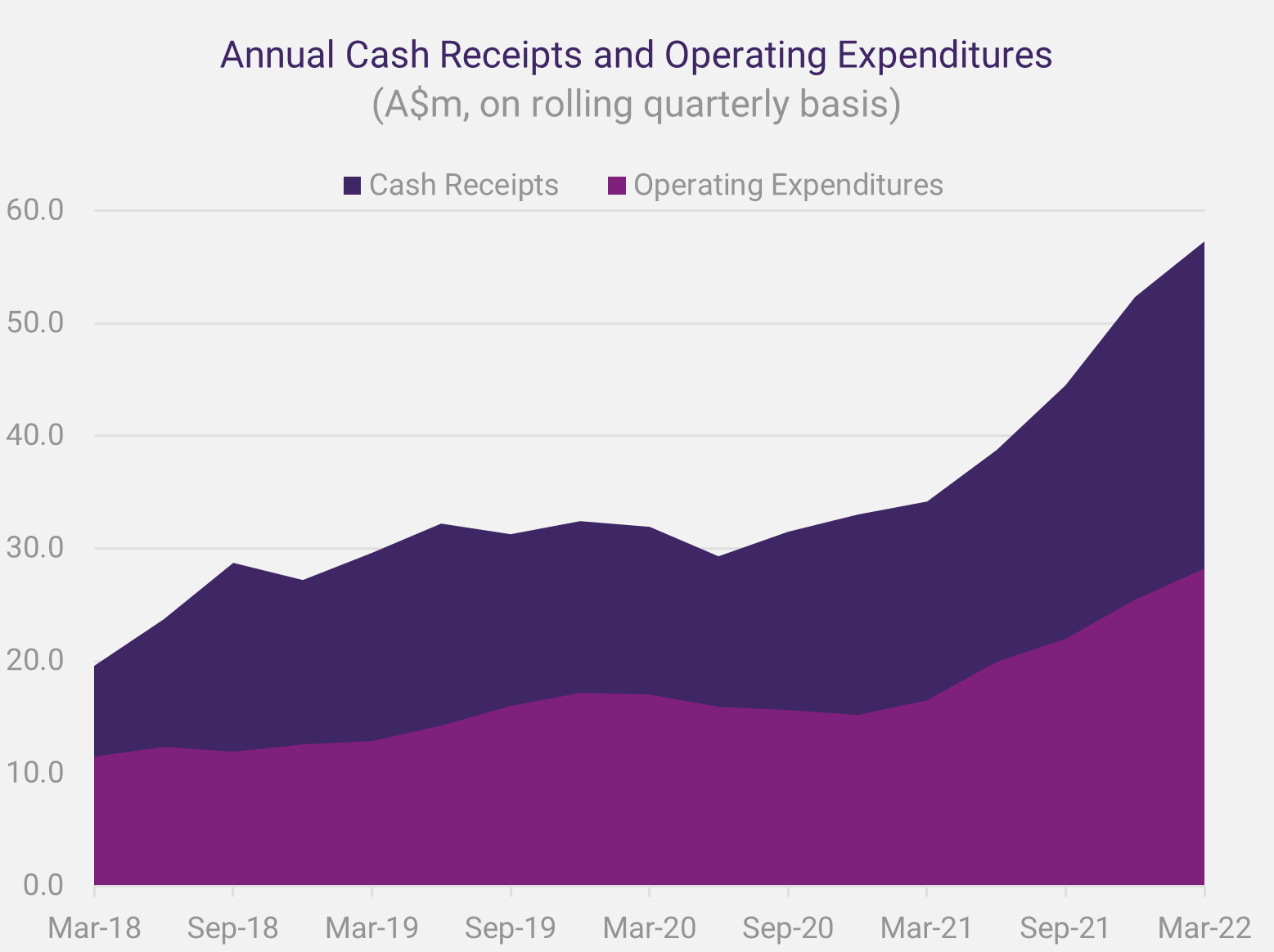
As we met with investors, directly and through meetings hosted by independent analysts and investment banks, we received consistently positive feedback on the Company’s approach to its resource management and reported cash position.
EU/US Roadshow
The majority of CLINUVEL’s shareholding is based outside of Australia, requiring the Company to engage with investors at a global level. At the time of writing, I am undertaking an extensive European and American Roadshow to connect face-to-face with existing and potential shareholders. This involves investor meetings and conference presentations in Switzerland, the UK, and the USA.
In the past, face-to-face briefings to shareholders have been held regularly and these have also helped to fund the research and development expenditures of the Group. CLINUVEL’s preference is to maintain relationships through regular face-to-face contact, as this results in deeper and more productive ongoing relationships. Whilst we managed to communicate effectively during COVID shutdowns and restrictions on movement over the past two years by using available technology effectively, we have always been keen to meet and interact with our investors face-to-face. Fortunately, highly-vaccinated populations and improved control of COVID-19 in a range of countries where our shareholders are located now allow us to resume the face-to-face contact that has been so beneficial in the past.
On 12 May we presented to longstanding shareholders and interested investors in Basel, Switzerland. Many shareholders present had invested in CLINUVEL for more than 10, and up to 17, years. The discussions with shareholders were inspiring to CLINUVEL personnel as they conveyed their ongoing commitment to the Group and our planned expansion. This solidification of their investment horizon drives us forward to progress our objectives to develop the Company into a diversified and sustainable pharmaceutical group focused on melanocortins. Meetings were also held with investors in Zurich and the UK. In the USA, I attended the H. C. Wainwright Global Investment Conference in Miami, presenting to the conference on 25 May and meeting investors and professional service providers. In New York, multiple meetings have been held with existing and potential investors and my visit culminates in attendance and presentation to the Jefferies Healthcare Conference on 08–10 June.
CLINUVEL is committed to further briefing sessions with shareholders where they are located around the world. A schedule of briefings is being formed and will be shared in the next News Communiqué.
The discussions with shareholders were inspiring to CLINUVEL personnel as they conveyed their ongoing commitment to the Group and our planned expansion. This solidification of their investment horizon drives us forward to progress our objectives to develop the Company into a diversified and sustainable pharmaceutical group focused on melanocortins.
Managing Director’s Comments
(Dr Philippe Wolgen, Managing Director)
Global Operations
The last quarter has been marked by an evaluation of our operations, as the Group continues to expand. A first conclusion is that COVID-19 has dramatically changed the concept of full-time employment, also affecting our companies across the continents. As is well reported in public press, I too believe that we have entered a new era when desk workers, employees mandated to do much of their desktop work are no longer expecting to work five days per week in the office. From our discussions with staff, the balance has shifted to two to three days working from the office, and the remainder of the week from home. Naturally, this choice is not given to those who work in laboratories and are required to be involved in dexterous labour, such as development and manufacturing.
The pertinent question for a pharmaceutical is how to balance the interface with employees in this modern era, how to rethink the activities and how much are we losing in working from home?
The key lies in planning, structuring, and ensuring clear objectives and timelines are in place for teams and divisions to adhere to. For now, CLINUVEL will operate on the basis of two to three days in the office, while the manual work will continue five days per week on the floor. Obviously, the question which follows from this practice is how much one should be spending on corporate offices, since these will become underutilised. These questions are being answered as we speak.
For now, CLINUVEL will operate on the basis of two to three days in the office, while the manual work will continue five days per week on the floor.
One thing is sure, the pandemic has changed expectations for a long time. In the midst of adapting to these changes, we monitor total factor productivity, looking at the mix of labour and capital, and here we have seen a constant during 28 months of restricted activities.
Swiss Investor Meeting, Basel, 12 May 2022
At times when equity markets suffer greater losses, and as analysts vary in their views as to the global sell-off in markets, I believe these periods serve midcap and specialised companies to rethink their communication with investors, and the financial community as a whole. Let’s step back in time (and expand on the comments made above) to provide the experience gained.
As the Company was facing its existential liquidity crisis in October 2005, we sat down to think of alternative ways of presenting the CLINUVEL story to new investors. Against convention, we self-organised and hosted meetings in the major financial centres in the world, presenting CLINUVEL to select groups of investors, who had historically shown interest in the sector. During an interval of 12 months, we presented directly to specialised investor groups in Vienna, New York, London, Paris, Frankfurt, Sydney, Zürich, and Geneva. At the time, it was the right occasion to invite feedback from the attendees on the proposed strategy to take afamelanotide to market, given years of unsuccessful attempts. The Company obtained invaluable responses, and unexpectedly was offered the chance to raise up to A$70 million dollars just weeks after the conclusion of the program. The two issuances were entirely driven by investors’ demand.
At the beginning of this quarter, as the rampage continued to spread across equity markets, in particular affecting life sciences, Board and management revisited the Company’s communication with global investors to raise further awareness and visibility. As we are aware that the appetite to talk about pharmaceutical and biotech companies has been waning ever since Q4 2021, we decided to both work with a number of investment banks as well as return to the earlier format of concentrated meetings with global investment groups. We have reverted to a focused investor relations program for 2022–2023, starting on 12 May in Basel, hosting a “Soirée d’Investissement” for Swiss investors.
We kick-started the comprehensive program in Basel, as a larger percentage (>12%) of CLINUVEL’s register is currently held by Swiss investors and most of these are long-term shareholders who invested in 2006 and 2007. Local banks and prospective clients, as well as existing Swiss shareholders, attended the meeting during which three themes were presented:
- investor relations (Mr Malcolm Bull);
- the activities of the Communications, Branding & Marketing team (Mrs Frances Myers); and
- general operations and strategy (by myself as Managing Director).
We subsequently released to markets Strategic Update IV (SU-IV), following SUI-II-III, a series which started in October 2020.As the CLINUVEL presentations drew to a close, a number of questions were posed. Although, all information provided has already been in the public domain, to gain an idea of the range of questions, we provide a verbatim summary here:
Question 1: “Will CUV continue its growth path?”
Answer 1: “CLINUVEL doesn’t provide financial guidance at this stage, there are too many uncontrollable factors which can influence day-to-day operations and outcomes. In managing the supply chain, our tasks are diverse, but one has still limited control over some of its parts, and the past decade we have been managing the business risks successfully as much as we can. Looking back, there have been many occasions when the Company had been facing critical decisions, but my observation is that the responsible managers and third-party contractors have been able to find solutions at these times. CLINUVEL is certainly not alone in facing complex problems, most of our peers in the sector will face similar problems if they decide to manage the critical element of drug development and commercialisation themselves.
“One of the keys to CLINUVEL’s growth has been the collective attitude to navigate unforeseen cliffs along the journey. In keeping this resolve over a long period of time, one builds value and grows. Whether growth continues, is dependent on our ability and willingness to solving problems within our control, plus the resources to weather macro events, such as we are facing at present.
As time passes, the Company is coming into a stronger financial position.
“For those who have followed the Company longer, it may be apparent that we express not taking the current successes for granted, and I am committed to see this awareness across the Group as one factor to help us mature further.”
Questions 2: “Are the expenditures going to increase over the next few years?”
Answer 2: “CLINUVEL had published, in November 2021 its Five-year forecast expenditures, totalling A$175 million, starting at financial year 2021. We are nearing the end of the second year, and we are tracking well, not exceeding the projections.
“One tries to get maximum output with relatively limited resources, there is no shame in attempting to do this. At the end, the smarter investor groups, but nowadays also larger healthcare insurance groups, critically ask how much the Company has spent on R&D, and here the feedback on CLINUVEL is unexceptionally positive.”
Question 3: “Will the CEO continue to put his passion in this company, what happens if he decides to step away? Is there a successor within the Company?”
Answer 3: “Today we have shared with you disappointing percentages in our sector, as investment banks and exchanges communicate to us that the majority of listed biotechs and pharmaceutical companies do not generate revenues and do not reach profitability. The principal objective was to take CLINUVEL to the minority of performing pharma companies, build a business around an asset which had failed for more than two decades, and that goal has been attained. The objective has shifted to expanding into a diversified group with multiple pharmaceutical assets and complementary products, and we are in the process of doing this.
“As to my enthusiasm for the business, I am enthused and motivated by the prospects of this company, but there will be a time when younger, better, more skilled professionals will take over the baton; my task is to get the Company to that point. The Board carefully made an inventory of views from shareholders, consultants and colleagues and concluded that it wanted me to continue one more term at CLINUVEL. For this to occur, we needed the team to stay together, and after lengthy discussions at home, I agreed a new contract until 30 June 2025.
“The Board is mandated to identify a successor in a timeframe such that a transition period is in place for my responsibilities to be handed over. As many of you who have been able to gauge from my past, I tend to start and finish to attain success. The same applies to the CUV puzzle, which is not yet completed. So yes, I have a lot of passion for this business, a vision to realise our ambition within the remaining three years.”
Question 4: “Why is CLINUVEL pursuing a cosmetic market, will it cannibalise SCENESSE®?”
Answer 4: “Well, that decision has multiple grounds.
“First, the medical community took 20 years to adopt the concept of hormonal photoprotection, that is systemically protecting the total skin surface against the insult of invisible and visible light. Introducing novel technology in a medical community always meets resistance, then we faced an overly concerned regulatory community. Data, compelling arguments, and consistency of our teams eventually overturned the stagnant opinions of decision makers and prescribing physicians.
“So, CLINUVEL became the first company to launch an endogenous protective agent, and its by-product activates melanogenesis, simply speaking, providing a Mediterranean bronze glow as a biomarker for effectiveness of the hormone: a visible indication that the product works on skin cells. In parallel, we focused all our resources on safety, we went out of our way to get dosing, frequency, length of exposure and selective prescribers. To more or less, guarantee safe use.
“Second, in having done all that work, we discovered that physicians were still recommending patients to use broadband sunscreens during intense summer months. In 2015, we recruited organic chemists, pharmacists and asked key questions as to the wider application of our knowledge and technologies. If the ingrained behaviour of physicians, dermatologists and organ transplant physicians is to keep recommending sunscreens as an adjuvant and despite the efficacy of SCENESSE®, why couldn’t they recommend our own labelled products complementing the innovative prescriptive hormone?
“So, we spoke at length with experts, dermatologists, and photobiologists. Could CLINUVEL not develop its own next generation polychromatic products, mineral based UV and HEV filters, which would preserve our oceans, products to be suited for those at highest risk of photodamage? Thus, we set out to do market research and found that a sizable segment had not been covered, the highest risk populations of contracting skin cancers, solar damage. There were three categories which had seemed unattractive to the cosmetic players and, for a number of reasons, these three populations have remained unaddressed in public campaigns. That is how the concept found its roots.
“The first product line is a new generation polychromatic solar protection, that is covering both UV and HEV exposure for those living and spending time under extreme conditions. Our teams will disseminate much information during our online campaigns. The second and third product lines will contain a combination of peptides, hormones, and anti-oxidative agents to assist DNA repair and aid pigmentation.
“There is logic and sequence in building the B2C business, first one builds an audience, tests the newly established market and targets specific channels. Then one adjusts, fortifies, and launches the second, third member of the family and so forth. Undertaking this as a pharmaceutical company is rare, both a challenge and exciting. However, the OTC lines very much belong and fit to our foray and leadership in photomedicine.
“Keep in mind our unusual but innovative odyssey, first we focused on EPP, patients suffering from absolute light intolerance, then XP patients affected by a deficiency in DNA (NER) repair and succumbing from multiple skin cancers, and shortly complementing it with non-prescriptive products to protect against the invisible and visible spectrum of light. This forms the trilogy we spoke about, EPP, XP and Highest Risk populations.
“In short, no, our leave-on products will complement, not cannibalise SCENESSE®.”
Question 5: “Are the products going to be different than found on the market, will these tan, how can CLINUVEL compete?”
Answer 5: “The products are different than existing comparables, the differentiation is found in its origin, ingredients, formulations, target groups and claims. The second and third products aim to maintain melanogenesis, acting on skin cells. We will reveal more of the aspects upon launch.
“Competition in the dermatocosmetic sector is high, and we are fully aware of the risks of distribution, marketing, and building followers in the market. Nothing ventured nothing gained, and we regard the risk to be relatively low to the overall business. We believe that a different approach to the market is worth exploring, since there is an obvious hiatus.
“The positioning of the products, its origin, target groups, the realisation that these come from CLINUVEL’s expertise in the field of photomedicine are all factors made to matter. We have established a specialised team to execute, again more to come in due course.”
Question 6: “Will analysts understand CLINUVEL’s dual strategy, and could it affect the rating of the Company?”
Answer 6: “That is an excellent question. The core business is and will remain pharmaceuticals. The Healthcare Solutions division can grow into a sizable business, but for now we focus on building a new market of populations at highest risk of solar damage and skin cancer, a market which cosmetic companies traditionally do not address en masse.
“Our teams will spend considerable time with market participants and analysts to explain our strategy, most of all expanding the visibility of CLINUVEL.”
Following the Basel meeting, feedback from banks, fund managers and investors has been unexpectedly and overwhelmingly positive. In evaluating the outcome, time and resources required, CLINUVEL is planning to continue a focused investor program, and over a period of 17 months, it intends to host 14 face-to-face briefing events around the globe:
| Location | Period | Theme |
|---|---|---|
| Basel | 12 May ‘22 | Strategic Update IV |
| Monaco | September ‘22 | Financial Year 2022 results |
| New York | October ‘22 | Strategic Update V |
| Sydney | October ‘22 | Strategic Update V |
| Melbourne | October ‘22 | Annual General Meeting |
| Frankfurt | December ‘22 | Operational Update |
| London | February ‘23 | Half Year Results |
| Singapore | March ‘23 | Strategic Update VI |
| Zurich | April ‘23 | Quarterly Results (Jan-Mar) |
| Paris | June ‘23 | Operational Update |
| Geneva | September ‘23 | Financial Year 2023 results |
| Monaco | September ‘23 | Financial Year 2023 results |
| New York | October ‘23 | Strategic Update VII |
| Sydney | October ‘23 | Strategic Update VII |
CLINUVEL is planning to continue a focused investor program, and over a period of 17 months, it intends to host 14 face-to-face briefing events around the globe.
Summary and Conclusion
CLINUVEL’s positive performance and progress continues, despite the deterioration in the world economic environment. The Company’s resilience reflects years of strategic focus to deliberately accumulate cash reserves to provide a buffer to adverse events and to be as self-reliant as possible. CLINUVEL has continued to generate net cash inflows during the quarters of FY2022, even as expenditures have increased to expand the business. CLINUVEL’s excellent fundamentals position us well to benefit from the turnaround in the economic cycle and more positive market sentiment, when it occurs.
CLINUVEL’s excellent fundamentals position us well to benefit from the turnaround in the economic cycle and more positive market sentiment, when it occurs.
In the meantime, we continue to focus on our objectives to build a diverse and sustainable pharmaceutical group and this commitment is reflected in the update in this news communiqué covering ongoing commercial operations and the progress being achieved with the expanded clinical program and work towards OTC product launch. You can expect regular updates on progress through the wider variety of communications now employed.
The re-opening of the world for travel enables CLINUVEL personnel to engage directly with more stakeholders where they are located. We are taking advantage of this to re-connect face-to-face with stakeholders around the world. During May and June, we have met investors across Europe and the USA, including presentations of CLINUVEL’s story to two prominent conferences. The feedback provided to CLINUVEL has been encouraging with a recognition of the value that has been added over the years and expressions of fortitude to remain patient during the current cycle to benefit from CLINUVEL’s excellent foundations when the cycle turns.
We wish all stakeholders ongoing safety and progress in their objectives.
Malcolm Bull, Head of Australian Operations & Investor Relations
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD.
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products; the COVID-19 pandemic and/or other world, regional or national events affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical products; competition for our products, especially SCENESSE® (afamelanotide 16mg), PRÉNUMBRA® or NEURACTHEL®; our ability to achieve expected safety and efficacy results in a timely manner through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, Israel, China and Japan of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE®, PRÉNUMBRA® or NEURACTHEL® which may lead to it being unable to supply its commercial markets and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology and consumer based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; our ability to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2021 Annual Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on preliminary and uncertain forecasts and estimates is available on request, whereby it is stated that past performance is not an indicator of future performance.
Contact
Level 11, 535 Bourke St
Melbourne, 3000 Vic,
Australia
+61 3 9660 4900
+61 3 9660 4909