CLINUVEL Communiqué II
| Melbourne, Australia, 31 March 2022 | ASX: XETRA-DAX: Level 1 ADR: |
CUV UR9 CLVLY |
Dear Shareholders, Friends
Introduction
(Mr Malcolm Bull, Head of Australian Operations & Investor Relations)
The world has become increasingly uncertain as war in Europe has exacerbated the ongoing overhang from COVID-19, building inflationary pressures, concern of higher interest rates and slower economic growth. During March, both the US Federal Reserve and the Bank of England raised their official interest rate benchmark by 0.25%, and further increases are expected. Forecasts of world economic growth for 2022 are also expected to be revised downward as inflation rises, particularly due to significantly higher energy and commodity prices, and the diversion of resources to an unwarranted war.
At CLINUVEL our path has been set and we continue to grow commercial operations and progress the expanded clinical program, notwithstanding the difficult environment, to build a diversified and sustainable pharmaceutical company.
In this communiqué, we cover several areas of the Company’s activities, specifically:
- the rationale for building a melanocortin specialty pharmaceutical group;
- progress in key clinical programs;
- our quality assurance approach and framework;
- review of key achievements in commercial affairs;
- recent developments in communications and investor relations; and conclude with
- insights into CLINUVEL’s business approach and future from Dr Wolgen.
At CLINUVEL our path has been set and we continue to grow commercial operations and progress the expanded clinical program, notwithstanding the difficult environment, to build a diversified and sustainable pharmaceutical company.
…, we have built unique expertise in specific fields which enables us to translate scientific understanding into pharmaceutical and healthcare products.
Building a Melanocortin Specialty Pharmaceutical Group
(Mr Lachlan Hay, Director of Global Operations)
CLINUVEL’s mission as an organisation emphasises our desire to translate scientific concepts and breakthroughs into commercial products, with a focus on those products which assist patients and broader audiences who lack alternatives. In short, we identify those with a high need and develop novel, scientifically based solutions. While this approach could be applied to many endeavours, we have built unique expertise in specific fields which enables us to translate scientific understanding into pharmaceutical and healthcare products.
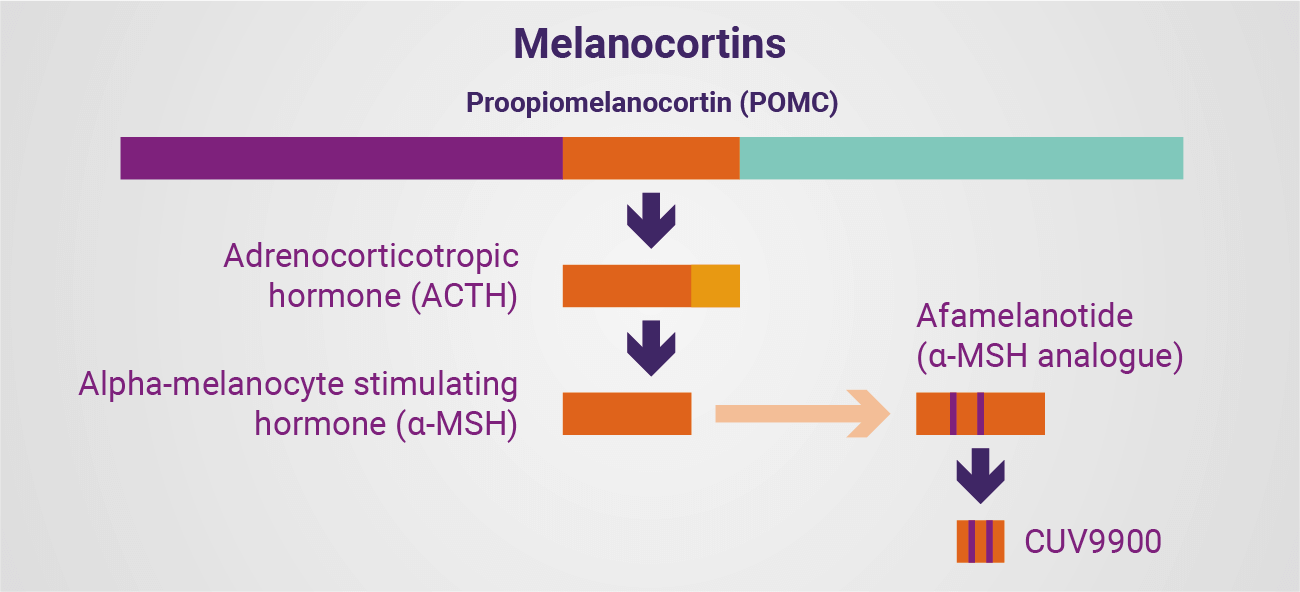
Central to this knowledge, accumulated over decades, is a world-leading expertise in the role of melanocortins, their analogues and specific formulations (finished presentation forms) tailored to human physiology. Perhaps the most reported of the functions of the melanocortin 1 receptor (MC1R) are those which are most visible to the human eye: melanogenesis. Naturally occurring alpha-melanocyte stimulating hormone – and its analogues, including afamelanotide – bind to the melanocortin-1 receptor on the pigment producing cells in skin, the melanocyte and keratinocytes, to stimulate the production and transport of melanin. In addition to the obvious dermal darkening effect of melanin, the pigment plays a photoprotective role, protecting skin from light, ultraviolet (UV) and high energy visible light (HEV) damage.
We are not discussing for now the important effects of afamelanotide on MC4R and its potency as a hormone, but leave this to the scientific team for another day.
It has been increasingly clear after two decades of experimentations, that a natural pathway to activate human cells is preferred and, in the long run, the only safe way to do so.
In addition to melanocortins, a number of synthetic drugs are able to activate melanogenesis, although not via the natural pathway. It has been increasingly clear after two decades of experimentations, that a natural pathway to activate human cells is preferred and, in the long run, the only safe way to do so. Deviations from these physiological pathways give rise to concerns around short- and long-term side effects. For example, cAMP agonists are recognised to affect most cells in our body and increase hypertension, while a new synthetic small molecule is reported to cause discolouration of the palms, soles, and alarmingly, in the eyes. These effects clearly indicate that the synthetic route is not the natural and biological pathway of melanogenesis being followed in order to assist the body.
Thus, a key strength in developing human melanocortins and their known analogues is that the pathways activated are recognised by the body’s own cells, are generally well known, and have considerable scientific understanding to justify their expanded use. Staying with a hormonal stimulation is the only safe way to go.
At their simplest, the melanocortins are a family of hormones, bioactive peptides and their analogues derived from proopiomelanocortin (POMC), a 241 amino acid polypeptide produced in the pituitary gland. These derivatives are able to bind to five specific melanocortin receptors (MC1R-MC5R) on cells throughout the body to then exert their effects. Natural adrenocorticotropic hormone (ACTH) is a long derivative of POMC, consisting of 39 amino acids, which is – in some instances – further shortened to 24 amino acids as a synthetic compound. The hormone binds to the MC2R to then exert its effects.
…, a key strength in developing human melanocortins and their known analogues is that the pathways activated are recognised by the body’s own cells, are generally well known, and have considerable scientific understanding to justify their expanded use.
Alpha-melanocyte stimulating hormone (α-MSH) comprises the first 13 amino acids of the ACTH sequence and binds with varying affinity to MC1R, MC3R, MC4R and MC5R to exert its effects. While best known for melanogenesis (the defensive tanning effect), α-MSH is also understood to have a broader role, given the presence of its main receptor – MC1R – on cells throughout the body and indicating the potential for manmade analogues of the natural hormone to be administered as therapeutics. Indeed, the real value of α-MSH is its effect on a great number of human cells, affecting vascular, nervous, and internal organ systems, and DNA affected by UV/HEV. This potent hormone is able to reduce fluid formation and stimulate a host of responses when used well and in moderation. Understanding this potential, and how to explore it, allows one to develop therapies.
This is exactly the approach CLINUVEL is taking with afamelanotide and other molecules. By analysing the early-stage pre-clinical knowledge around both the natural (biological) α-MSH and its analogues, and combining this with the Company’s expertise in targeting cells via specific doses and formulations, we arrive at the potential to treat a range of indications.
This is how we can translate the use of a peptide-based drug currently successfully treating patients with an ultra-rare disorder (erythropoietic protoporphyria, EPP) for use in DNA repair (xeroderma pigmentosum, XP), repigmentation (vitiligo), and central nervous system disorders (arterial ischaemic stroke, AIS). These programs are now being aggressively pursued by the Company to determine which patient groups can benefit most from the treatment.
…, the real value of α-MSH is its effect on a great number of human cells, affecting vascular, nervous, and internal organ systems, and DNA affected by UV/HEV. This potent hormone is able to reduce fluid formation and stimulate a host of responses when used well and in moderation.
Beyond afamelanotide, but staying within the same family of hormones, CLINUVEL’s analysis has further led to our more recently announced ACTH program, where an opportunity arose not only to meet a looming market gap for existing patients, but to add to various therapeutic arsenals with our approach. Here, we plan to not only commercialise ACTH formulations (NEURACTHEL® Instant and NEURACTHEL® Modified-release), but also broaden the therapeutic use of this hormone in identified patient groups with a high unmet clinical need.
Put simply, CLINUVEL’s particular expertise and knowhow – in melanocortins, formulations, dosing, cellular signalling and clinical execution – is allowing us to translate and grow scientific knowledge to help more patients and individuals among broader audiences.
Earlier this month we announced an update on the ACTH program, with cGMP manufacturing underway and development and validation work in progress. In parallel, our team in Singapore is advancing a number of melanocortin-based projects to build our future pipeline, both as pharmaceuticals and healthcare solutions. The table below provides a status of the various formulations:
Put simply, CLINUVEL’s particular expertise and knowhow – in melanocortins, formulations, dosing, cellular signalling and clinical execution – is allowing us to translate and grow scientific knowledge to help more patients and individuals among broader audiences.
CLINUVEL’s Melanocortin Portfolio
| Product | Dosing | Formulation | Status |
|---|---|---|---|
| SCENESSE® (afamelanotide 16mg) |
Adults | Solid | Commercial |
| SCENESSE® ENFANCE (afamelanotide) |
Paediatric 12–17 | Liquid | In development |
| PRENUMBRA® Instant (afamelanotide) |
All ages | Liquid | cGMP production |
| PRENUMBRA® Modified (afamelanotide) |
Adults | Liquid | In development |
| CUV9900 | Adults | Topical, leave on | In development |
| NEURACTHEL® (ACTH) Instant | Adults | Liquid | cGMP development and validation ongoing |
CLINUVEL’s Active Clinical Development Program
| Afamelanotide | |||
|---|---|---|---|
| Indication & addressable market | Melanocortin therapeutic approach | CLINUVEL progress | |
| Erythropoietic protoporphyria – adults Prevalence: 1:140,000 in Europe, US |
Prevention of phototoxicity (anaphylactic reactions and burns): photoprotection, anti-oxidative | SCENESSE® approved in US, Europe, Australia, post-authorisation studies ongoing | |
| Erythropoietic protoporphyria – paediatric | Prevention of phototoxicity (anaphylactic reactions and burns): photoprotection, anti-oxidative | SCENESSE® ENFANCE in development. | |
| Variegate porphyria (VP) Prevalence: 0.3-2:100,000 |
Prevention of phototoxicity (blistering burns): photoprotection, anti-oxidative | Phase II study in planning. | |
| Xeroderma Pigmentosum (XP) Prevalence: 1:1,000,000 |
DNA Repair, photoprotection, anti-oxidative | Phase II studies underway in XP-C, XP-V, and disease-free subjects. First results due 2022. | |
| Vitiligo Est. 45 million patients worldwide, first targeting skin types IV-VI in North America |
Repigmentation, activation of melanocyte stem cells | Phase II combination therapy pilots complete, afamelanotide well tolerated (n=55). Phase II monotherapy pilot to commence in USA in 2022. | |
| Stroke (AIS) 15 million strokes per annum, 85% of patients are ineligible for current treatment |
Vaso-activation, anti-oxidative, anti-oncolytic | Preliminary Phase II results show afamelanotide well tolerated (n=6). Full results due 2022. | |
Clinical Programs
(Dr Pilar Bilbao, Head of Clinical Operations)
An overview of key developments in CLINUVEL’s expanded clinical program is provided below:
Arterial Ischaemic Stroke (AIS)
Earlier this month we released preliminary results from our CUV801 study, CLINUVEL’s first to evaluate the use of afamelanotide in patients suffering from AIS. We are strongly encouraged by this initial top-line data, and it is with great anticipation that we now work diligently to capture, review and analyse the final results of the study. It is of no less importance that the first time a melanocortin has been used in critically ill patients has stood up to the test, this is of great interest to the medical and scientific community.
The primary endpoint of the CUV801 study is safety, as it should be at this stage in the program. Unlike in other programs, however, there is a further rationale for the focus on safety due to the impacts that existing stroke therapies can have on exacerbating the extent of stroke patients’ injuries. The unimaginable tragedy of worsening the condition of these patients needs to be carefully assessed when using a new drug in this group; we have withstood the first test of safety.
As a brief recap, when a patient suffers an ischaemic stroke, part of their brain is deprived of oxygen and nutrients by a clot in an artery, killing a core of tissue around the injury and placing a larger region of the brain at risk of damage. Known as the penumbra – Latin for “shadow” as this is how the region appears on imaging scans – tissue in this affected region can be recovered if a stroke can be treated, or further damaged if blood flow is not restored. Brain damage as a result of a stroke is generally irreversible, leading to permanent disability and a lifelong handicap for patients. The period following the immediate stroke is thus critical to the overall clinical outcomes for stroke patients as a treatment seeks to minimise the overall impact of the stroke.
The current “gold standard” treatments for ischaemic stroke focus upon the chemical dissolution and/or physical removal of a clot, known as thrombolysis and thrombectomy, respectively. Intravenous thrombolytic therapy (IVT) relies on the administration of recombinant tissue plasminogen activator (alteplase or tenecteplase; rt-PA) to dissolve a blood clot within a blood vessel. While often very effective in dissolving clots and subsequently restoring blood flow, IVT can greatly increase a patient’s risk of fatal intercranial haemorrhage if administered at too high a dose or too long after stroke onset. In general practice it is recognised that IVT must be administered within 4.5 hours of stroke onset if it is to provide the greatest benefit to a patient (this leads to very low uptake rates of IVT, around 12% of hospital admissions in the UK, for example). Thrombectomy also presents a risk to patients. This process of mechanical clot removal can be conducted within a longer therapeutic window, but the overall risk increases with time. The removal process is delicate, requiring specific training, and risks rupturing vessels within the brain if the clot cannot be easily dislodged. It can also only be used for clots which occur in more easily accessible, larger blood vessels, limiting the overall eligible patient population. Thus, while these treatments can be very effective, they carry sizeable risks and are limited in their use.
We are strongly encouraged by this initial top-line data, and it is with great anticipation that we now work diligently to capture, review and analyse the final results of the study.
The CUV801 study administered up to four doses of afamelanotide to patients over the course of eight days. This extensive therapeutic window was chosen to evaluate the impact of treatment intervention at different stages of the patients’ injuries and understand whether afamelanotide would be well tolerated. It is thus very encouraging that no reports of drug related side effects, “adverse reactions”, were received and we can now further consider the optimal dosing windows for afamelanotide with a level of comfort from this first study.
The efficacy data analysed as at day 8 of the study are equally encouraging. The National Institutes of Health Stroke Scale (NIHSS) is an internationally recognised and validated tool used to evaluate neurologic functioning and impairment after stroke onset and throughout the recovery phase. Deployed by trained clinicians, the NIHSS comprises 15 tests to assess consciousness, language, neglect, visual-field loss, extraocular movement, motor strength, muscle control, speech, and sensory loss. Scored on a scale of 0-42 (higher scores meaning greater disability), the tool allows a consistent approach to quantifying stroke severity both within an individual patient during clinical care, and across cohorts of patients in a study environment. It is generally recognised that a reduction in NIHSS scores of ≥4 point is clinically meaningful, reflecting a positive outcome for a patient.
The efficacy data analysed as at day 8 of the study are equally encouraging.
Of the six patients treated under the CUV801 protocol, five saw a meaningful improvement in their symptoms as captured on day 8 of the protocol. Tragically, the sixth patient suffered a second, fatal, stroke while in hospital on day 5, which was determined to be unrelated to their treatment with afamelanotide by both the neurologists at the Alfred Stroke Unit and CLINUVEL’s safety board.
The CUV801 protocol continues patient monitoring for up to 42 days post stroke, with data captured throughout this period. As the patients complete the monitoring period, we expect to learn more about the longer-term impact of the intervention with afamelanotide, both in terms of patient safety and clinical outcomes. In the interim our team will continue to review data as it is generated in this open-label study and apply these learnings to the next steps in our stroke program, with further studies now being planned. Thus far it has been rewarding for our teams to learn that our work is helping to translate the findings in pre-clinical environments into a potential treatment for patients, and we are excited to see what the full CUV801 results will teach us later in the year.
Of the six patients treated under the CUV801 protocol, five saw a meaningful improvement in their symptoms as captured on day 8 of the protocol.
DNA Repair
The DNA Repair Program is another translation of our melanocortin technology which continues apace across three protocols. Most recently, the CUV152 study treated the first xeroderma pigmentosum variant (XP-V) patient. This protocol is the first to focus on the treatment of XP-V patients, with the program to date having focused on XP-C and disease-free subjects in CUV156 and CUV151, respectively.
XP is a rare life-threatening inherited disorder characterised by defects in the body’s own system to repair UV and light-induced DNA damage. XP-V patients’ defect is different to other XP patients in that it is not the nucleotide excision repair (NER) process that is affected. Rather, XP-V patients’ cells cannot replicate UV-damaged or oxidised DNA, due to a defect in the enzyme DNA polymerase eta. In the absence of this enzyme, other polymerases (which do not recognise the lesions) may replicate the DNA. These replications can then create errors and enhance mutagenesis. In addition, XPV cells show increased sensitivity to UVA light, resulting in oxidative stress and impairing the overall health and function of the cell.
The primary objective of the CUV152 study is understanding the impact of afamelanotide therapy on specific markers of DNA damage and repair. Based on current discussions with the expert centres conducting the study, we expect preliminary results from the study in 2022.
The ongoing CUV156 study continues to recruit and treat XP-C patients, with results expected later this year. In a parallel protocol, the CUV151 mechanistic study is looking at the impact of afamelanotide treatment on DNA damage and repair in an unaffected population to determine whether differences in the intervention can be identified and the knowledge fed back into our programs.
The DNA Repair Program is another translation of our melanocortin technology which continues apace across three protocols.
CLINUVEL’s Quality Assurance Approach and Framework
(Dr Azza Hamila, Head of Quality and Drug Safety)
Quality Assurance (QA) in pharmaceuticals is a broad concept which covers all aspects that could have an impact on the quality of prescribed pharmaceutical products. In short, QA measures are designed to ensure that the prescribed medicine competently provides the desired effect, is safe for patients, and complies with the governing regulations. QA is also a mindset within an organisation, one with a focus on continuous improvement of systems and their rigorous implementation.
CLINUVEL’s global Pharmaceutical Quality System (PQS) encompasses all CLINUVEL licenced and non-licenced entities, as well as its Research, Development & Innovation Centre, VALLAURIX. Driven from the UK and Ireland where, the European drug licenced entities are located, our PQS consists of the coordination of several quality through processes, with the aim of producing finished products of the highest quality:
CLINUVEL’s global Pharmaceutical Quality System (PQS) encompasses all CLINUVEL licenced and nonlicenced entities, as well as its Research, Development & Innovation Centre, VALLAURIX.
Good Manufacturing and Distribution Practice (GMDP)
Good manufacturing practice (GMP) ensures products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by the marketing authorisation (MA) or product specification. This applies to the whole supply chain, from sourcing of amino acid peptides for an active pharmaceutical ingredient (API) through to manufacturing, packaging, quality control testing and batch “release” of a product for use in a specific market. Companies can also be selective in their approach, reflecting their specific business needs. Similarly Good distribution practice (GDP) requires that medicines are obtained from the licensed supply chain and are consistently stored, transported, and handled under suitable conditions, as required by the MA or product specification. Product traceability and authenticity is key here.
CLINUVEL’s European entity, based in Dublin, is the legal holder of the SCENESSE® marketing authorisation but also the holder of a Manufacturing and Import Authorisation (MIA) licence overseen by the Irish national competent authorities, the HPRA. The HPRA, on the behalf of the European Medicine Agency, regularly inspects the site to ensure compliance with the GMP and GDP. Importation of the implants into the European Union (EU) and global distribution is managed through the European subsidiary.
The UK subsidiary, CLINUVEL (UK) LTD, also holds a MIA, as well as a Wholesale and Dealer Authorisation (WDA) licence used to import and distribute SCENESSE® into the UK. The MHRA, the UK’s supervisory authority, regularly inspect our UK site for GMP and GDP compliance, enabling us to distribute SCENESSE® in the UK after Brexit.
Supplier Qualification System
Due to the globalisation of pharmaceutical supply chains, the expectations of manufacturers and sponsors to control activities and materials provided by their suppliers have increased. It is the responsibility of CLINUVEL to ensure that its global suppliers provide services and materials that are fit for purpose throughout each product’s lifecycle, from product development through commercial distribution. CLINUVEL’s QA has implemented a supplier qualification and management program build on four major steps: develop user requirements, supplier due diligence and selection, quality assessment, and ongoing monitoring e.g., through audits. This allows us to assess new and existing suppliers, as well as amend our supply chain as needed.
It is the responsibility of CLINUVEL to ensure that its global suppliers provide services and materials that are fit for purpose throughout each product’s lifecycle, from product development through commercial distribution.
Quality Risk Management
A robust quality risk management programme is essential to ensuring the quality of pharmaceuticals by anticipating the identification and control of potential quality issues during development and manufacturing. CLINUVEL’s QA has implemented an ongoing risk-based approach throughout all its processes and procedures. This involves the identification, assessment, control, communication, and review of the risks to each of the processes. By taking this approach, we are being proactive rather than purely reactive, to prevent or reduce undesired effects and protect patient safety. Risk is often thought of only in the negative sense, but a risk-based approach can also help to identify opportunities and thus promote continuous improvement.
CLINUVEL’s QA has implemented an ongoing risk-based approach throughout all its processes and procedures.
Deviations and Corrective and Preventive Actions
Minor and temporary variations are part of a functioning system. Proactive handling of deviations, and minimising their recurrence, is critical in the functioning of a pharmaceutical quality management system. When a deviation to a regulation, a process, a procedure, or to the registered specifications is identified, an appropriate level of root cause analysis is applied during the investigation using Quality Risk Management (QRM) principles. Appropriate corrective actions and/or preventative actions (CAPAs) are then identified and taken in response to the results of the investigation. The effectiveness of such actions is monitored and assessed, always in line with QRM principles.
Controlled Document System
Documentation is the key to QA compliance and ensures traceability of all development, manufacturing, testing and distribution activities. CLINUVEL’s global documentation system ensures effective policies, protocols, procedures, work instructions, specifications, test methods, reporting documents, training modules and technical documents are made available to all the Company’s employees.
Training and Continuous Improvement
Well-qualified employees are an essential part of GMDP as part of overall QA. As such, requirements for qualifications, training and development of all employees involved in GMDP relevant operations must be met to ensure that employees can aptly perform their assigned tasks according to their position.
CLINUVEL’s new employees are placed on an induction training programme adapted to their role and assigned tasks and responsibilities. A continuing GMDP training program is established by the QA department for all relevant employees, with the involvement of senior management, to ensure that all aspects required by the GMDP standards are covered. Managers are responsible for identifying required training needs of their staff on an ongoing basis and particularly, during annual appraisal evaluations.
The performance of CLINUVEL’s PQS is regularly reviewed, with the involvement of senior management, to identify opportunities of continual improvement of the products, processes and the system itself. Continuous improvement is critical to the success of the Company since it contributes to the consistent delivery of safe and efficient pharmaceutical products with the appropriate quality attributes. It also creates opportunities for innovation where the QA teams across the Group are constantly reflecting and analysing data looking for trends. As they learn from the data, they also generate new ideas to deliver further improvements and efficiencies.
Continuous improvement is critical to the success of the Company since it contributes to the consistent delivery of safe and efficient pharmaceutical products with the appropriate quality attributes.
CLINUVEL’s Commercial Affairs
(Ms Antonella Colucci, Vice President Commercial Affairs)
Focus of Commercial Affairs
Our department of Commercial Affairs plays an active role to support the general operations of the Company. We have built and maintained contacts and relationships with service providers, hospitals, and doctors essential to support CLINUVEL’s commercial supply. This work is part of a consistent approach which I have led for 11 years. This is most relevant in the diligence exercised to build solid relationships with all parties and individuals involved in the distribution of SCENESSE® (afamelanotide 16mg) to treat adult EPP patients.
Not only the physicians, but also pharmacy staff and procurement and finance professionals at each European Porphyria Expert Centre where EPP patients are treated are critically important. Our team liaises regularly with the active centres to receive feedback on treatment provided, the expected number of patients, and treatment patterns. SCENESSE® is a high-value product, manufactured in small batches and therefore the delivery of every single implant must be planned accurately so the patients’ needs are met in a timely fashion. We are aware that some EPP patients need to take time off from work or family life to travel to their treatment centre and we strive to make sure that there is no delay in the delivery of SCENESSE®. Clinical pharmacies also play a pivotal role in the distribution of SCENESSE®. They are the first point of contact of our team as they submit orders and receive the product under controlled conditions. Our focus is to be a reliable partner and we try hard to answer any question they have on logistics and distribution within the same day.
The real difference for SCENESSE® distribution to work smoothly is the productive and positive relationships developed through the meetings of our team with physicians, pharmacists but also regulatory bodies over the years, including visits to treatment centres and networking at scientific events. This provides the “human touch” that has proven invaluable to ensure the smooth distribution over the years and more recently, in the difficult COVID-19 operating environment.
The real difference for SCENESSE® distribution to work smoothly is the productive and positive relationships developed through the meetings of our team with physicians, pharmacists but also regulatory bodies over the years, including visits to treatment centres and networking at scientific events.
I review below key achievements in commercial affairs and current initiatives that will shape the Company’s future commercial growth.
A Decade of Swiss Distribution
Switzerland was one of the first countries in the world to allow access to SCENESSE® for the prevention of phototoxicity in EPP adult patients. Since May 2012, SCENESSE® has been distributed in Switzerland pursuant to specific bylaws and reimbursed by the Swiss Krankenkassen (health insurers).
CLINUVEL’s key focus has been to support the medical team in their efforts to treat EPP patients. Our experience in Switzerland has proved that a collaborative approach to working with payors best facilitates the access of patients to treatment. We knew that EPP is (and remains) a poorly understood disorder and we have always been open to discuss it with Swiss insurers to raise awareness of the disease and help them understand the real clinical value of SCENESSE® for EPP patients. This approach has proven successful and all Swiss EPP patients demanding treatment are granted access.
From a clinical perspective, Swiss and Italian physicians published the first observational study on SCENESSE® in EPP in 2015 and more recently, Dr Anna Minder has published another observational study demonstrating the protective effect of afamelanotide on the liver (as reported in News Communiqué I – 2022).
Our experience in Switzerland has proved that a collaborative approach to working with payors best facilitates the access of patients to treatment.
Controlled Distribution Network
Only adult patients with a confirmed diagnosis of EPP can be treated with SCENESSE®. CLINUVEL is committed to distributing SCENESSE® in accordance with the provisions of the Risk Minimisation Plan (RMP) approved upon centralised marketing authorisation in the European Union (EU) on 22 December 2014. To minimise the risk of off-label use, we established a rigid controlled distribution programme to ensure SCENESSE® treatment reaches the correct patients through European EPP Expert Centres.
Since June 2016, when SCENESSE® was first launched in the EU, distribution across Europe has been fully managed by the CLINUVEL team. Our Standard Operating Procedures do not include distribution through local pharmaceutical wholesalers, which would add a variable of uncertainty to our controlled access program. Orders are sent by Expert Centres to our team and processed internally. Our external distributors deliver the product following our team’s detailed instructions. SCENESSE® is delivered exclusively to hospital pharmacies and in no case made available to retail pharmacies. Once delivered, the pharmacist, who has also been previously trained by the CLINUVEL team, will confirm delivery by returning a signed and dated acknowledgement of receipt and a copy of the temperature readout. As SCENESSE® must always be refrigerated, the temperature readout will be immediately controlled by our Quality Assurance team. In case of out-of-range temperature excursions, our staff will reach out to the site and advise on the suitability and safety of the product for patient use.
CLINUVEL is committed to distributing SCENESSE® in accordance with the provisions of the Risk Minimisation Plan (RMP) approved upon centralised marketing authorisation in the European Union (EU) on 22 December 2014.
Key Initiatives
We have an ongoing program to distribute SCENESSE® to EPP patients in new jurisdictions. This includes a range of countries in Europe where we seek to progress agreements on the reimbursement of the cost of treatment with key payors. This objective also extends to Australia. In other regions of the world, we are working to address the regulatory approvals necessary to distribute SCENESSE® and specifically mention both China and Japan which have EPP populations in need of treatment. In China, our relationship with Winhealth Pharma progresses with focus on a named patient program to establish a Chinese patient dossier. Here we seek to combine local data with the safety and clinical benefit captured globally to approach the Chinese authorities for consideration of approval to distribute SCENESSE®.
We have an ongoing program to distribute SCENESSE® to EPP patients in new jurisdictions.
Communications and Investor Relations
(Mr Malcolm Bull, Head of Australian Operations & Investor Relations)
Recent Announcements
The Company has maintained an active flow of information on the progress of the business since the start of the year with a range of key announcements and webinar/webcasts, as detailed below:
| Date | Announcement |
|---|---|
| 10 January | Presentation to H.C. Wainwright Bioconnect Conference |
| 17 January | Enrolment Completed in CUV801 Stroke Study |
| 24 January | SCENESSE® continued in Germany |
| 27 January | News Communiqué I – 2022 |
| 31 January | Quarterly Activities / Appendix 4C Cash Flow Report |
| 14 February | Expansion DNA Repair with Second Study |
| 16 February | Advice of Operations Update Webcast (held on 23 February) |
| 23 February | Appendix 4D Half Year Report |
| 28 February | CEO Letter (and webcast) |
| 15 March | afamelanotide in Stroke (AIS) – Positive Preliminary Results |
| 17 March | ACTH Manufacturing Update |
| 30 March | First XP-V Patient Treated in Third CUV DNA Repair Study |
| 31 March | News Communiqué II – 2022 |
All of CLINUVEL’s announcements are available on the CLINUVEL website and CLINUVELNews. More specifically, announcements to the Australian Securities Exchange are available on the investor pages of the CLINUVEL website.
Recap of Financial Results
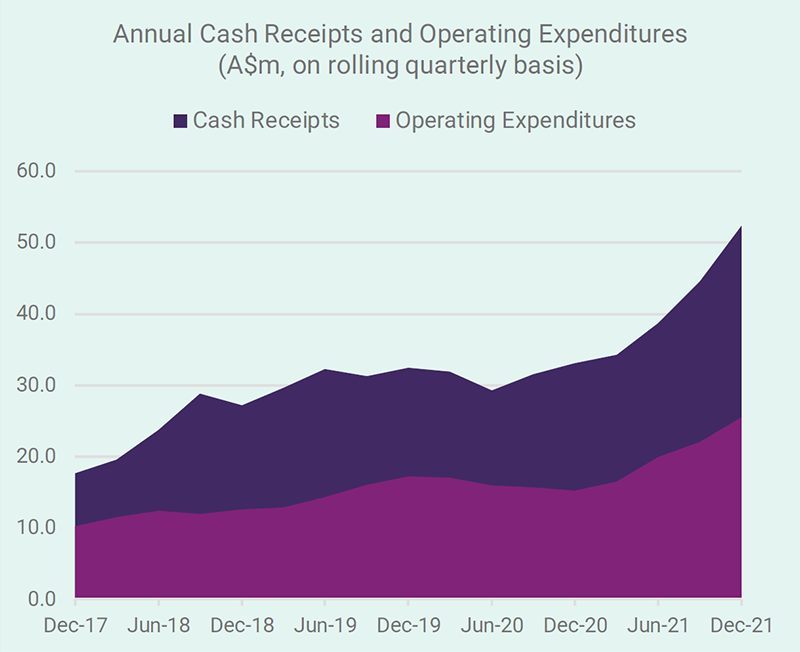
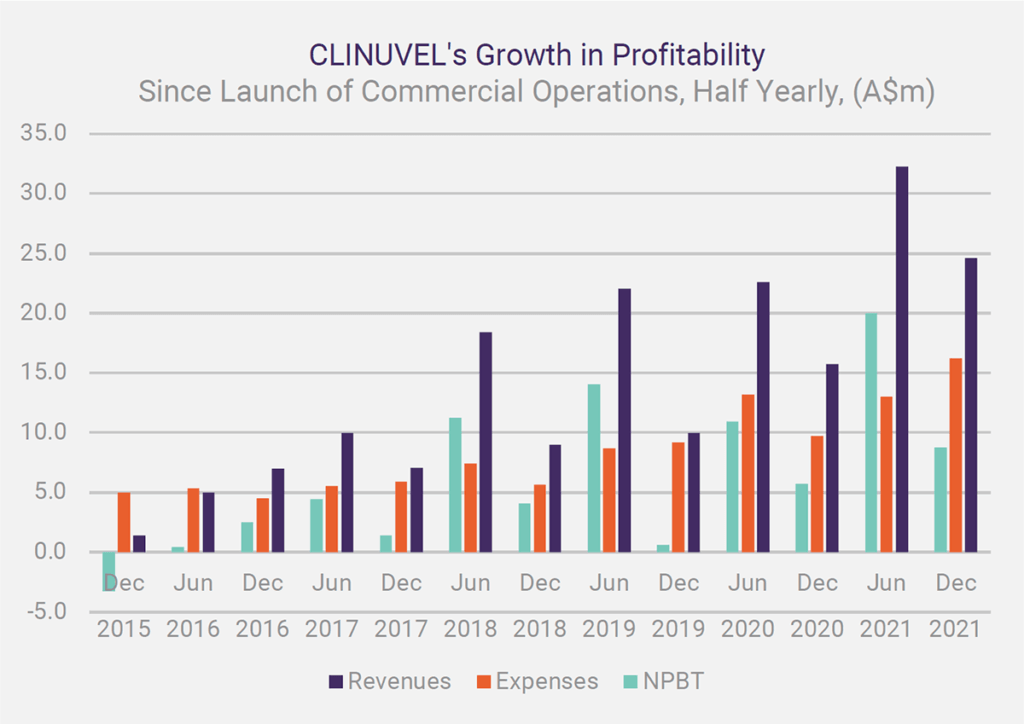
Since News Communiqué I – 2022, we announced the December quarter 2021 cash flow outcome and the financial results for the half year ended 31 December 2021. Record cash receipts for a December quarter and record revenues and operating profit for a December half year indicate the healthy financial performance of the Company. The distribution of SCENESSE® in Europe, the USA and Israel continues to grow and generate positive cashflow to cover the expenses necessary to expand the business and boost cash reserves, to just under A$99 million as at 31 December 2021. The benefit of this position, particularly in the current environment, cannot be understated.
Record cash receipts for a December quarter and record revenues and operating profit for a December half year indicate the healthy financial performance of the Company.
CFO, Darren Keamy and Director of Global Operations, Lachlan Hay answered key stakeholder questions in Operations Update I – 2022 on the half year results. Mr Keamy and I also made presentations to institutional investors directly and through independent analyst hosted meetings. We also answered the questions of other shareholders on a range of issues relevant to the business in one-on-one meetings and emails.
Each of the independent research analysts issued updated reports following the half year results. We appreciate their ongoing work and research, particularly the increasing recognition of the incremental value being established through the progress of the expanded clinical program.
CEO’s Letter and Webcast
The CEO provided an overview of CLINUVEL’s market position, financial management and growth in a letter and webcast in late February / early March. Dr Wolgen commented that the reduction in the share price since late September 2021 is in direct contrast to the positive ongoing performance of the business with the achievement of record cash receipts, revenues, and operating profit in the latest reporting periods. He also put the significant rise in expenses (of 67%) in the half year to 31 December 2021 in the context of the total planned expenditures of A$175 over five financial years (2021 to 2025) announced in the November 2021 Annual General Meeting. This was well received by stakeholders who also expressed unanimous support for the Company’s position on the inexcusable invasion of Ukraine by Russia.
We will continue to advise stakeholders of the progress of the clinical programs when new data becomes available. The next financial data on the Company will be the March quarter 2022 cash receipts to be released by the end of April 2022.
…, the reduction in the share price since late September 2021 is in direct contrast to the positive ongoing performance of the business with the achievement of record cash receipts, revenues, and operating profit in the latest reporting periods.
The Why?
(Dr Philippe Wolgen, Managing Director)
A prominent US shareholder, comparing CLINUVEL to his other life science investments, asked me to openly explain our approach to the pharmaceutical business and rather differentiating strategy. This was a good question to receive, since it is believed that many US investors seem to be hardly aware of CLINUVEL’s successes. I happily take the opportunity to share our balanced views on the business and future.
Whilst general statements are not necessarily applicable at all times, there are nonetheless three repetitive themes influencing our management of the Company:
- First, we distinguish between essential versus non-essential services, goods, and products during economic contractions. This bifurcation has served as a rough guide to identify those companies which are able survive and attract continuous market attention, and those who periodically struggle.
- Second, we tried learning from past economic crises (in particular, the GFC), so taking on a pre-emptive, rather than reactionary, demeanour.
- Third, we carefully monitor valuations within our sector and ascribe realistic scenarios during both high versus low markets, with particular reference to the largest pharmaceutical market, the US. Thereby, we view balance sheet strength as one of the parameters taking long-term uncertainty away from investors and positively influencing valuations.
At times when market volatility dominates, we believe there is even greater need for a global view on economies, while it allows these observations to be brought back to CLINUVEL. So, let us start from a bird’s eye view.
If physical restrictions worldwide, owing to a single viral outbreak, would have seemed improbable three years ago, we have seen how airlines and many other businesses centred around discretionary services were brought to the brink of bankruptcy. In some European corridors, high-street retail became extinct, and peoples’ inability to physically interact caused paralysis of recreational and hospitality businesses. In its immediate wake, the Ukrainian crisis compounded fear of economic dislocation and fuelled inflation.
Well, the pandemic for one has shown the power of patient and physician demand. In CLINUVEL’s case, porphyria patients have been willing to travel, to risk breaking lockdowns, and seek treatment under isolated conditions. This unusually strong willingness to obtain treatment, seen in both the US and EU, cemented to our teams that afamelanotide was being perceived by end users themselves as more or less an “essential treatment” allowing them a life in freedom even under the most adversarial conditions. This clinical observation is not only valuable – even without actually having planned for this phenomenon to occur – but heavily influences our decisions and future direction.
Second, we strongly believe in the cyclical nature of businesses, and in a dynamic pharmaceutical pricing environment there is always a risk of disruption of a steady economic state affecting innovative pharmaceutical products. Healthcare insurers are continuously seeking new avenues to renegotiate drug prices, and in our view anticipating possible future changes is mandatory for every pharmaceutical company. Therefore, in order to make the business recession proof, we adhere to a capital adequacy rule preparing CLINUVEL for unforeseen gyrations. Again, our financial strategy has avoided further equity or debt financing during past market corrections. While I would not advocate a change in this management, new times, new conditions and opportunities may well change our views. In general, feedback to date has been very positive on a pre-emptive strategy to manage with fiscal prudence.
…, in order to make the business recession proof, we adhere to a capital adequacy rule preparing CLINUVEL for unforeseen gyrations.
Third, in monitoring and benchmarking CLINUVEL against other melanocortin companies and specialty pharmaceuticals, but also among a myriad of other innovators in our sector, valuations matter for us to keep perspective. Amongst many variables, we take into account dilutionary rate, addressable markets and balance sheet. Price to book and price-earnings ratios provide some clues as to how CLINUVEL tracks against others, through both good and bad markets.
A Few More Words on CLINUVEL’s Approach
I have the benefit of speaking in two capacities, as a larger shareholder of CLINUVEL, sharing your very interests, and as daily manager. Let’s look at the current corporate story, which has evolved over time from these two perspectives.
There is overlap in my dual viewpoints. As a shareholder, I would want to understand – as much as I am able to from the outside – the risks of the venture, and its management following a methodical program to eliminate these risks. How does one continuously adapt to addressing new unforeseen risks? As an investor, I would want to see growth, early profits and new projects, cum quoque investments.
As a fellow shareholder, I wish to see a life science group able to manage long cycles, innovate and try its maximum to manage these aeons without dilution of equity at each and every occasion. The cost of equity is exemplified in many companies, which eventually have come to succumb or actually do not realise commercial value from their product launches.
I have the benefit of speaking in two capacities, as a larger shareholder of CLINUVEL, sharing your very interests, and as daily manager.
As managers, risks are at the forefront of our mind, and this attitude has led to CLINUVEL’s current position.
In sum, as valuations have come down and life science companies are currently under strain, a conventional route would have seen the Company carrying six months of cash, requiring it to spend most of its time presenting the story to raise funds. As a shareholder, I ponder what the Company would have looked like now and could look like into the future, amidst the uncertainty prevailing? As a manager, I wonder how many managers would have stayed in the Company under these circumstances and how much talent and value would have walked out the door?
To round up, I see it as my principal task to provide hope and fuel ambition for younger generations of new staff for them to carry the Company forward. One piece of advice I impart to our managers is to always keep a final option to oneself, keep the best project close to your chest and play it out when cataclysm plays out.
Having shared with you two viewpoints, I leave you with the notion that the Company has not been in a better financial position, while the sudden rise of CLINUVEL’s share price during September 2021 and decline in subsequent months, bears no relationship to our commercial progress and development.
…the Company has not been in a better financial position, while the sudden rise of CLINUVEL’s share price during September 2021 and decline in subsequent months, bears no relationship to our commercial progress and development.
Summary and Conclusion
There are many roads leading to Rome, as one says. We have chosen a deliberate and focused strategy implemented over many years leading to CLINUVEL’s strong position.
CLINUVEL progressed from a concept with one drug, to its use for systemic photoprotection, transitioning to assisting repair of DNA damaged skin, and is progressing further to a B2C market for populations at highest risk of solar damage and skin cancer. We translated our knowledge and technology to diseases of the central nervous systems and added new molecules belonging to the same family of melanocortins. In doing so, the Company established an infrastructure bringing most skills in-house and built a strong financial position.
Investing in life sciences is knowing that one relies on all events playing out in favour of development. Running a biotech equals seeking the edge of failure while making sure one can control many of the internal risks. There is no absolute guarantee CLINUVEL will continue to do well, but the dots over the past decades can be connected to draw a trend line.
We wish all stakeholders ongoing safety and progress in their objectives.
Malcolm Bull, Head of Australian Operations & Investor Relations
There is no absolute guarantee CLINUVEL will continue to do well, but the dots over the past decades can be connected to draw a trend line.
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD.
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products; the COVID-19 pandemic and/or other world, regional or national events affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical products; competition for our products, especially SCENESSE® (afamelanotide 16mg), PRÉNUMBRA® or NEURACTHEL®; our ability to achieve expected safety and efficacy results in a timely manner through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, Israel, China and Japan of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE®, PRÉNUMBRA® or NEURACTHEL® which may lead to it being unable to supply its commercial markets and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology and consumer based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; our ability to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2021 Annual Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on preliminary and uncertain forecasts and estimates is available on request, whereby it is stated that past performance is not an indicator of future performance.
Contact
Level 11, 535 Bourke St
Melbourne, 3000 Vic,
Australia
+61 3 9660 4900
+61 3 9660 4909