CLINUVEL Newsletter V
Dear shareholders, friends,
As the world battles on to control the spread of COVID, we provide some colour on the past financial quarter and the financial year end. We take stock and review the progress of the Company to provide context.
With pleasure we received the figures for the last quarter of the financial year 2020 (ending 30 June) which exceeded our internal projections. We had little time to dwell on these results as the end of financial year audit was due immediately after. This audit is a large-scale exercise of intense weeks for all staff, a time when independent auditors quiz and analyse all key audit matters of the parent and subsidiaries. All operations and financial reports are scrutinised within the Group and all governance matters are up for review.
As iterated in the News Communiqué III, we stress-test processes when it comes to financial management of the Group. Every year under current management – without exception – all reports have come out clean and without material defects or significant observations. A Chief Financial Officer very seldom gets acknowledgement for their conduct, but in Darren Keamy we have a “safe pair of hands managing the till”. This seamless management provides CLINUVEL’s Board and shareholders much comfort in a world rife of deceit and off-balance sheet activities. I view the execution of pharmaceutical projects at the CFO’s behest. Financial expedience allows strategic planning which aims, in turn, to reduce (as much as possible) business risks in a sector already filled with uncontrollable third-party risks and unforeseen delays.
The financial year end 2020 has revealed a year-onyear increase of 5% in revenues and addition of 23% in cash balances. This performance is in line with our aim to front load our ability to grow the Company and withstand times of global economic headwinds. With the number of patients and treatments increasing, the start of distribution in the US, and new projects emerging, there is very little we could have asked from our team in terms of output.
While supply chains had come to a halt in April, export countries Germany, Japan and China have since increased their manufacturing output. The slowdown in raw materials and finished products has hardly affected CLINUVEL, whereby the European Medicines Agency has shown a willingness to speed up processes relating to product release. I view CLINUVEL’s progress in the context of the economic uncertainty arising in the Eurozone, the United Kingdom and Australia, all of which have now plunged into recession.
While the United States is posting -8% negative growth over the quarter with 11.4 million job losses since February, we have seen a modest recovery of the US economy the past weeks, whereby it remains uncertain whether all sectors will follow at the same pace. At the time of writing US unemployment ranges from 8.4% to 11.4% depending whether one accounts for the people who have recently applied for social welfare. In Europe, the picture is equally grim: the Eurozone accounted for 7.2% unemployment with the prospect of an economic recovery bleak as we witness a surge in COVID numbers. We remain in unknown territory; hope and incertitude dictate all nations. The possibility of a vaccine by mid-2021 and whether it would reach sufficient people in time will determine the ability of governments to relax stringent measures.
Overall, the inflation target of 2% set by central banks (read European Central Bank and the Federal Reserve) is not being achieved. A high inflation rate would theoretically provide the central banks and governments more room to adjust interest rates, as we witness bottom level rates across the G8 countries. Cheap credit is available in the markets and those companies able to service the loans longer term are in a reasonably good position to steer clear of the crisis. As the economic commotion surrounds us, I see bright lights for CLINUVEL, subject to all of us remaining in good health and allowing us to work our way through the multiple projects evolving simultaneously. Working through a series of crises have made us resilient, and we will prevail this time once again.
CLINUVEL’S OBJECTIVES
The FDA approval in October 2019 has made room for our teams to reassign the various research projects, allocate more resources to these activities, and invest time and manpower in the most urgent developments. The past quarter was important in that we have seen the emergence of PRÉNUMBRA® and the start of the DNA Repair Program. The use of afamelanotide in UV-induced DNA damage represents a serial progression towards the generation of final scientific evidence demonstrating benefit. The full context and significance of this program will be explained in a strategic update in October; in essence it brings together all parts towards multiple objectives.
The pandemic has certainly had an effect on European and American hospitals and their capacity to initiate new clinical studies. This shut down impacted the exploration of afamelanotide in our next sponsored indication (medical application), which had been planned for July. We now patiently wait for the all-clear from the local competent authorities before we can start a new chapter in a life-threatening condition.
All in all, corporate incentives are congruent across the entire Company, affirming that all are working towards the same long-term goals. I see an environment created for newly joined talent to develop and thrive, and I have little doubt that our cross-functional teams will meet the goals we had set and shared in November 2019.
DNA REPAIR PROGRAM
On 14 and 15 September we resumed the polyptych aim of using afamelanotide as a regenerative therapy in patients affected by UV and visible light insult.
After more than a decade of working towards trials in xeroderma pigmentosum (XP) patients, our teams were able to start the clinical program. In XP there is an exasperation from families worldwide to find any therapeutic means, let alone an agent to lessen the daily burden of their children unable to expose to light and sun. Time is of the essence for XP patients, since there is a high mortality in this group of diseases. XP is also one of the most disfiguring diseases one can come across, and to finally be able to use a systemic photoprotective drug is a scientific aspiration coming true. The preparation of the trial CUV150 is the longest I have witnessed owing to the regulatory and ethics approvals required, as well as the consent needed from patients and their families.
Generally speaking, hospitals and national authorities are very cautious and showing reluctance to subject XP patients to clinical trials, due to the severity of the disorder, and relatively short life expectancy. In 2006, I had learned firsthand about a US company which had failed in its attempt to treat these patients with a topical enzyme replacement therapy. Having studied the company’s pathway leading to the FDA rejection, CLINUVEL’s aim became firm and engraved. We were positive that the first results generated by afamelanotide had provided data showing reduction of photoproducts, and from this moment onwards our ultimate mission was to gather sufficient additional evidence to convince regulatory bodies and ethics committees to allow us to evaluate treatment for the XP population. The regulatory requisite was a set of compelling scientific data from SCENESSE® (afamelanotide 16mg)1 to make the following arguments:
(i) clinical safety mid- and long-term;
(ii) second set of clean toxicology data;
(iii) plausibility of clinical success in XP; and
(iv) systemic photoprotection in the UV high energy visible (HEV) light spectrum.
In the second quarter of 2020 we arrived at a position where all four criteria had been met. We had crossed the four-year mark post-authorisation, and clinical safety is now consistent with over 20 years of consecutive use of afamelanotide, 16 years in erythropoietic protoporphyria (EPP) patients and four years under real world conditions – predominantly in Caucasian (fair) skin types. With this incessant attention to safety, the XP pathway and final stage in the DNA Repair Program was opened.
Given the risk of high morbidity (accompanying illnesses) and mortality, clinical trials in XP patients are complex and need to be considered at length before decision makers can actually accede to the scientific concept. In principal, the discussions with the authorities centred around the gains expected from afamelanotide, balanced with the burden of the trial itself to the patients. Rightly so, judgements need to be made over a long period of time to ensure that none of the participants (authorities, pharmaceutical sponsor, patients and physicians) make a critical error. Each XP patient is screened multiple times, informed of the risks, and followed up prior to the start of the pilot study. Once the family and patient consent to the SCENESSE® therapy, the expert physicians commit to the trial and total care of patients in a multidisciplinary team. Transport to and from the centres is carefully planned, with blinded vehicles providing a barrier and minimising the risk of light and UV exposure. It is known that the risk of additional photodamage and skin cancers is 10,000-fold higher in XP.
In XP, we have started the dosing of afamelanotide 16mg to one male XP-C patient to monitor his tolerance to the drug product. The patient is clinically followed up by each day by medical staff, while daily assessments are made of safety and the effectiveness of systemic photoprotection. Skin biopsies provide histological (cellular tissue) information as to the pre-existing photodamage. The overall objectives in developing afamelanotide in XP-C patients are summarised best as the evaluation of the:
(i) safety in XP-C patients;
(ii) effects on the integrity of their skin (preexisting poikiloderma);
(iii) effects on photoproducts (following UV exposure);
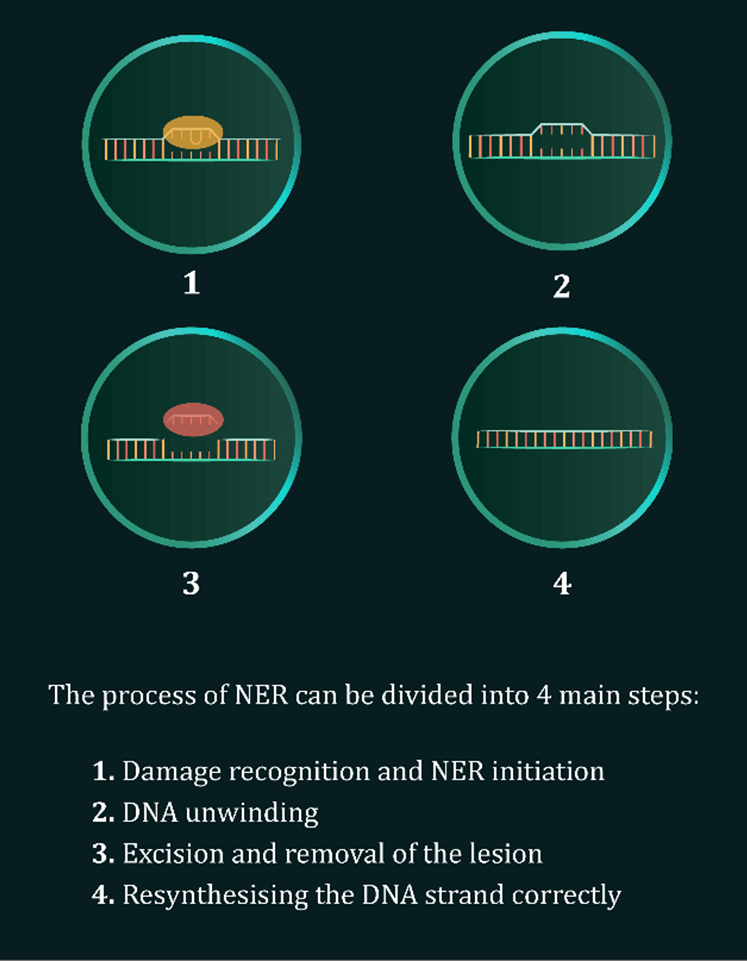
(iv) effects on nucleotide excision repair (NER); and
(v) clinical effects as a systemic photoprotective drug.
The NER pathway, activated following UV and HEV exposure, is explained in detail in our Scientific Communiqué III and aided by a recently released video and multiple postings from our communications team on social media platforms. During the next months, more information and news flow on the DNA Repair Program and associated themes are to be released.
USA DISTRIBUTION UPDATE
At the time of News Communiqué V being published, our US teams have issued training programs and accreditation to 22 EPP Specialty Centers across the US. Our target in April was to achieve 10 accredited Centers by Q4 2020, and, up to 30 Centers trained by Stage 2 of the US Distribution plan (June 2021). Pleasingly, Dr Teng and her staff are ahead of schedule, and the team is aiming to ensure a broad geographical spread of active Centers across the US to accommodate patients. The commonality in our US approach is “the drug in the proximity of the clusters of US patients”. As a first impression, patients’ first clinical response to SCENESSE® meets expectations and safety data are similar to those observed in Europe over the years. To this extent, we have not received new and unexpected information when it comes to pharmacovigilance (medical monitoring of side effects). This month, we are due to supply an annual report to the FDA, summarising the most recent safety findings, with the next Periodic Adverse Drug Experience Report due in November.
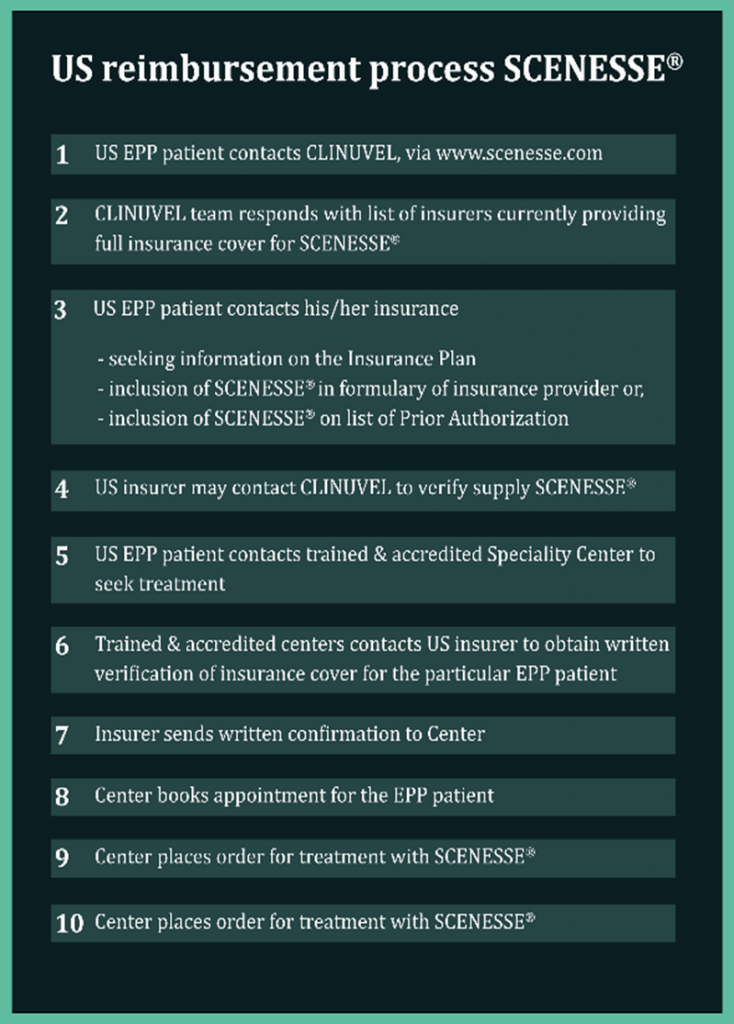
Interstate, agreements continue to be reached with US insurers for treatment coverage under Prior Authorization (PA); nevertheless, some insurance providers resort to lengthy exchanges with physicians on the reimbursement of the clinical consultation required for implantation of SCENESSE®. We observe that these discussions generally result in a satisfactory outcome for the healthcare providers and clinical staff members on reimbursement for their consultation time. The process of PAs can be time consuming for the first treatment, while second and subsequent treatment requests are handled faster and without queries. Each EPP patient has access to our US team for further personal assistance during the reimbursement process as often insurers request more product information.
Various medical institutions work from their own formularies, and two larger hospitals have recently included SCENESSE® onto their “in-house formulary”, a meaningful step nationwide in acknowledging the drug as standard of care in EPP. The current formularies serve as a future reference for other institutions seeking confirmation of uniform pricing.
SCENESSE® is designated as a specialty drug and listed as a Tier 4 prescriptive therapy administered in a medical setting (i.e. doctor’s office or outpatient hospital facility). Depending on the insurance plan taken out, healthcare plans may require co-payment from the insured EPP patients. However, there is an annual out-of-pocket maximum up to US$8,150 for medical treatment received under medical benefit. Our teams continue to work to ensure equitable treatment of US EPP patients.
VALLAURIX EXPANSION
With much pride, we opened our Singaporean Research & Development Center (VALLAURIX) on 31 August. As we are expanding our expenses on research and development, specific projects are now attracting new talent in Singapore. VALLAURIX is one of the few research companies in the region recruiting new staff.
New scientific staff are being added in areas of peptide chemistry and polymer science, advanced analytical chemistry, and regulatory Chemistry-Manufacturing-Control (CMC). While Global Quality Control remains coordinated from Europe, local QC staff are on site. Specific professionals in Regulatory Affairs have been added to bolster the teams. In terms of equipment, we expand our facilities ahead of requirements to enable the laboratories to fulfil all functions in-house. In working closely with the Singaporean Economic Development Board, we are securing the subsidies made available for the recruitment of local talent and capital expenditures.

Work on the molecules CUV9900, phimelanotide and parvysmelanotide is entering a new phase, while more analytical methods are being developed. As announced in July, with PRÉNUMBRA® we launch a liquid formulation of afamelanotide in-house to allow dosing flexibility of the hormone analogue afamelanotide for acute and systemic diseases. The second-generation product builds on scientific knowledge of the pharmacodynamic effects of afamelanotide and leverages on existing technology. As said, the objective is to commercialise an injectable liquid dosage form in a polymeric carrier, providing modified release of afamelanotide. The development of the new formulation is guided by the Quality by Design approach
PUBLIC AND INVESTOR RELATIONS
Since January 2020, key events in our calendar have been changed to virtual meetings. However, the intensity of communication with the financial community in Australia and the US has not decreased, gauging from our internal logs. Part of monitoring our internal output has been to record any incoming emails, calls and video conferences since 1 March. After six months we observe an increase in productivity as seen from frequency of communications and progress of projects. In a world condemned to working from home, I see our office staff bouncing back from an adversity many had never experienced before.
The positive financial results of the Company have been well received since these were achieved during the pandemic. Deliberately, we controlled increase in expenditures to support the growth and expansion of the Group. The commentary reflects the understanding of our longstanding strategy which has been consistently communicated over the years.
Positive feedback was also received from various shareholders on the declaration of the third consecutive annual dividend. The unfranked dividend of A$0.025 per share for the 2020 financial year was consistent with the 2019 financial year dividend, itself an increase of 25% on the first dividend of A$0.02 per share in 2018. At a time when many companies are unable to declare a dividend or have needed to reduce their pay-out ratio to allocate cash reserves to finance ongoing operations, the Board continued to show its appreciation of the long-term support of shareholders and the need to maintain healthy cash reserves in these uncertain times. This approach has been recognised in shareholder feedback received to date. The final dividend per share as of the record date of 04 September 2020 amounted to a distribution of A$1.235 million and was paid on 18 September 2020. This is a positive augmentation of the overall return to CLINUVEL’s investors
The Company has released announcements on the financial and operational performance and progress of the research and development programs in recent months. Recent operational news flow reflects the increase in R&D investment, including the announcement of PRÉNUMBRA®, the opening of the Research & Development Centre in Singapore and, this month, the DNA Repair Program. We have received support from many sources on our initiative to assist the XP patient population.
All the announcements of the Company are available on the CLINUVEL website, www.clinuvel.com, with the key announcements for 2020 listed in the table below for ease of reference.
|
Key Announcements to the Australian Securities Exchange – 2020, year to date |
||
|
Date |
Announcement |
Details |
|
16 January |
CLINUVEL Newsletter |
Communique I – 2020 |
|
31 January |
Appendix 4C – quarterly |
Cash Receipts, December quarter 2019 |
|
03 February |
Australian TGA Commences SCENESSE® Review |
Dossier to be reviewed under priority registration pathway |
|
10 February |
Request for FDA Guidance Meeting SCENESSE® in Vitiligo |
Request for Type C Guidance Meeting |
|
24 February |
CLINUVEL to Expand its Singapore Laboratories |
Laboratory expansion with Singapore Government grant |
|
26 February |
Appendix 4D Half Year Results |
Financial Report, half year to December 2019 |
|
27 February |
Corporate Presentation Half Year Results |
Focus on December 2019, half year results |
|
02 March |
Supply of SCENESSE® Unaffected by Coronavirus |
SCENESSE® and its excipients unaffected by coronavirus |
|
03 March |
FDA Meeting Confirmed to Advance SCENESSE® in Vitiligo |
Type C Guidance Meeting confirmed for 29 April |
|
12 March |
Chair’s Letter to Shareholders |
Chair’s Letter I – 2020 |
|
19 March |
CLINUVEL Newsletter |
Communique II – 2020 |
|
23 March |
US Distribution Update |
Corporate presentation on US distribution SCENESSE® |
|
23 March |
CLINUVEL to Launch SCENESSE® in USA in April |
Phased US treatment rollout planned |
|
16 April |
First US Patients to be Treated with SCENESSE® |
CLINUVEL’s innovative drug launched in USA |
|
23 April |
CLINUVEL Starts SCENESSE® Supply into China |
Agreement with Winhealth Pharma to launch pilot program |
|
28 April |
Appendix 4C – quarterly |
Cash Receipts, March quarter 2020 |
|
01 May |
FDA Type C Meeting for SCENESSE® in Vitiligo |
Type C Guidance Meeting held with FDA |
|
08 May |
Chair’s Letter to Shareholders |
Chair’s Letter II – 2020 |
|
19 May |
CLINUVEL Newsletter |
Communique III – 2020 |
|
20 May |
Presentation UBS Global Healthcare Conference |
Corporate update presentation to virtual conference |
|
05 June |
Presentation Jefferies Global Healthcare Conference |
Corporate update presentation to virtual conference |
|
13 July |
PRÉNUMBRA® – New Liquid afamelanotide Presentation |
Second formulation of afamelanotide |
|
22 July |
CLINUVEL Newsletter |
Communique IV – 2020 |
|
31 July |
Appendix 4C – quarterly |
Cash Receipts, June quarter 2020 |
|
31 July |
Corporate Update |
Corporate update presentation |
|
27 August |
Appendix 4E and Preliminary Final Report |
For financial year ending 30 June 2020 |
|
27 August |
CLINUVEL Full Year Results |
Fourth consecutive annual profit result |
|
27 August |
Declaration of Full Year Dividend |
Third consecutive annual dividend |
|
27 August |
Corporate Presentation |
Focus on 2020 full year results |
|
31 August |
Opening of VALLAURIX R&D Laboratory in Singapore |
New R&D facility opened in Singapore |
|
10 September |
SCENESSE® in DNA Repair |
CLINUVEL progresses innovative DNA Repair Program |
|
15 September |
First Patient Dosed in SCENESSE® DNA Repair Program |
Xeroderma pigmentosum (XP) patient receives treatment |
|
23 September |
CLINUVEL Confirms AGM Date |
Virtual AGM set for 11 November 2020 at 18.00 hrs AEDT |
|
23 September |
CLINUVEL Newsletter |
Communique V – 2020 |
We frequently brief prospective shareholders on CLINUVEL and explain the nature of the innovative biopharmaceutical company with its long-term strategic focus, stable and experienced management, and established a track record of positive cash flow and profitability. We ensure a measured distribution of returns to shareholders, and we maintain healthy cash reserves to self-finance the growth of business operations and the expansion of activities to treat multiple patient groups. We continue to attract interest from private and institutional investors to invest in CUV. Many of the interested institutional investors have a long-term investment horizon which fits the chosen business model and provides stability to focus on our core objectives. This interest is supported by the independent research coverage of CLINUVEL (listed on the CLINUVEL website), as well as the client facing personnel of investment banks and brokers that we regularly brief on the Company’s progress. We have welcomed new institutional investors during 2020 and, more broadly, can report the number of registered shareholders has increased by 36% to 5,449 over the year to 15 September 2020. The calendar of planned events is updated, noting the prevalence of postponed and cancelled events due to the coronavirus pandemic and our participation in virtual conferences.
We have maintained focus on attending these conferences as a way to reach a larger audience of potential investors and tell the CLINUVEL story and the compelling reasons to consider us as an investment. The conference presentations we announce to the ASX also serve to provide an update on the story and advise progress to existing and potential investors.
Some new conferences have been added to the planned events, subject to final confirmation.
In the coming weeks, we will provide a strategic update, finalise and distribute the 2020 Annual Report, and prepare for the virtual Annual General Meeting of Shareholders to be held on Wednesday 11 November 2020 at 18.00 hrs AEDT. More generally, we will continue to keep all stakeholders informed of the progress of the research and development program.
SUMMARY
Like many life science actors, we are facing an enormous challenge in current times, and thus far our group of companies is rising to the task. Operations, R&D and finance are all positively aligned in what I regard to be the most testing months of CLINUVEL’s existence. We set out to establish a Company for many years, to grow and offer staff job security while millions of people are losing their source of income. I want CLINUVEL to be a stand out among its peers and be a place which genuinely cares about its staff, known as a safe environment for professionals wanting to develop their individual talents, albeit under time pressure to collectively deliver results. It needs to be a place where professionals work along a set of incentives aligned with our corporate objectives.
The past eight weeks we have progressed on many fronts as the table of key announcements (above) shows. Value is being built and the Company is being noticed by an increasing pool of financial institutions and services. CLINUVEL grew its assets by 29% in the past year and continues to monitor its expenditures carefully, maintaining a lean operation.
The coming months are equally exciting as we gather more data on new products in development, as we learn about the progress in XP, and await the approval of new programs for afamelanotide.
I thank you all for your continued support and shared enthusiasm for the Company.
Philippe Wolgen
1SCENESSE® (afamelanotide 16mg) is approved in the European Union as an orphan medicinal product for the prevention of phototoxicity in adult patients with EPP. SCENESSE® is approved in the USA to increase pain free light exposure in adult EPP patients with a history of phototoxicity. Information on the product can be found on CLINUVEL’s website at www.clinuvel.com.
Download PDF