CLINUVEL Communiqué IV
Dear Shareholders, Friends
Introduction
With satisfaction, we look back at an eventful interval since we last communicated with you in News Communiqué III. Much progress was made on the pre-clinical, clinical, and commercial side of the Group.
On 29 July, we shared our quarterly cash flow performance (April to June) and the results were better than expected, given the restricted access to European hospitals who are still not working to full capacity.
This News Communiqué IV concentrates on a number of specific topics.
In this Communiqué IV, I also wish to delve into a recent question posed by a long-term a Swiss asset manager who quizzed the Company’s emphasis on safety and, particularly, why this appeared to be of such importance. We shall discuss this item in more detail as it directly links clinical to shareholder value.
Against these macro issues, sad news reached us about the most prominent and longest serving sector analyst, David Blake of Bioshares. David started Bioshares Analyses in 1998 with Mark Pachacz, and both are the most committed analysts of the sector in the Asia-Pacific.
Recently, David has suffered severe health issues, and I call all of you who have met David to send him your thoughts and well wishes. He is a formidable man, conscientious, knowledgeable and with a deep interest for our sector. Without David Blake and Mark Pachacz leading Bioshares, many companies would not have received attention and visibility in the sector and consequently, had the ability to raise finance and develop products for patients.
David is influential to CLINUVEL’s course and strategy, and has been a key party to our successes to date. While he had been critical of Epitan at the turn of the century, he motivated me to pick up the battle to get the drug to patients. He has rightly predicted CLINUVEL timelines, the seeding stage, the harvesting stage, and the expansion stage, where we are at present. Without the weekly publications of Bioshares and periodic input from David and Mark, we would not have had deeper insights on the failings, faux-pas and success stories of other companies, the dos and don’ts in the Asia-Pacific region.
It is unfathomable to see David on the receiving end of healthcare after spending three decades analysing and following the companies. The CLINUVEL team sends its love and affection to David’s family, Sonya and Alethea. We continue to send our prayers, wishes and energy to a great Australian man, who has much to contribute to our sector.
Financial Year Ended 30 June 2021
Our prudent and thorough approach to managing the business’ financial household has been a constant factor over the years. The energy put into the systems, processes and controls are the foundation of our financial team and their ability to execute a global commercial program. Today’s results are the best the Group has ever recorded and are based on years of preparation and discipline to navigate to a peer leading financial dashboard.
Today’s dials show, for FY2021, a 43% increase in total revenues to A$48.451 million. While a steep increase in expenses of 56% was already seen in FY2020, enabling us to keep a stable rate of expenses and reinvestments, we contained the increase in our expenses this year to 2%. Reinvestments occurred in new systems, technologies, new peptides, and manufacturing processes, while we grew our talent bases quite aggressively. Cost of integration during rapid expansion is not expressed financially, nevertheless a real factor.
What is the underlying reason CLINUVEL distinguishes itself from other companies in stressing the importance of safety of afamelanotide, a novel hormone introduced medical use?
FY2021 saw an increase in the number of treatment centres providing access to patients, the absolute number of patients receiving treatment, and the number of new patients seeking SCENESSE® (afamelanotide 16mg). The number of implant administrations rose, as well as the average number of implants per patient. This translated to a record net profit after income tax of A$24.728 million, an increase of 64% compared to the previous financial year.
These results show that annual predictions can be fruitless: our own internal anticipation had been lower, given the prolonged pandemic restrictions and uncertainty. The market conditions have been overcome by our teams creating novel avenues, working with decision makers, and taking the treatment access closer to patients. Under the UK team’s guidance, physicians, representatives, and national competent authorities were engaged in weekly conversations to find solutions for patients.
The priorities given to the business directly translate to profitability and hence total shareholder returns, for FY2021 recorded 28%.
Earnings per share continued to rise to A$0.50, while appreciation for shareholders are returned in the form of a modest dividend.
Clinuvel’s Emphasis on Safety
At the time of this piece going live, we are nearing a significant moment in time of having administered 10,000 implants of afamelanotide to various patient populations over the course of 16 years. The hormonal treatment translates to 70,000 drug exposure days with a remarkable stable and benign profile of adverse events (side effects). The most common side effects seen after SCENESSE® administration remain headaches and nausea the first 24 to 48 hours, fatigue, gastrointestinal discomfort, flushing and localised reactions at the implantation site.1 Generally speaking, the most credible and valuable testimonies on a drug’s safety comes from patients themselves, reporting the adverse events experienced spontaneously, or through their physicians or national medicines reporting systems. Sixteen years onwards, afamelanotide’s safety profile remains consistent as the database of long-term drug users demanding treatment year on year continues to grow.
Our frequent communication on safety of afamelanotide over the years requires an enumerated narrative. What is the underlying reason CLINUVEL distinguishes itself from other companies in stressing the importance of safety of afamelanotide, a novel hormone introduced medical use?
As the CLINUVEL case is being written by Columbia University (intended publication late 2022), there is a chronological course of events providing insight to the longitudinal attention to safety. As our teams had been asked by regulatory authorities to scrutinise the safety of the product beyond reasonable doubt, this attention would actually become one of our strongest arguments to decision makers, regulatory reviewers, insurers, and the medical community. The profile of afamelanotide and variety of diseased populations addressed would give us a competitive advantage over any other company developing an MC1R and MC4R agonist (drugs binding and activating melanocortin receptors 1-4).
At some point the suggestion was made that CLINUVEL was allegedly suspected of actually being one of the rogue laboratories distributing unregulated illegal substances.
In introducing a novel hormone for human use, we faced resistance from regulatory agencies worldwide repetitively questioning the Company’s ‘true’ intended use of the product. As several previous management teams had publicly announced plans to make afamelanotide available for the wider tanning market, CLINUVEL was thought to follow the same track. With the history carved in the minds of decision makers, regulatory reviewers of global agencies had refuted the possibility of pharmaceutical biomimicry triggered by effects of UV to skin, causing melanogenesis (darkening and body tanning). In plain English, the use of a systemic hormone to trigger a protective skin response was frequently rejected in scientific meetings on the basis of concerns of safety. And even today, our teams occasionally face stiff rejection of the medical concept, albeit the safety profile of SCENESSE® is now acknowledged as remarkably consistent and positive.
Parallel, in these days, the world woke up to more than 100 rogue laboratories emerging from Middle America to Europe and Asia, distributing the rogue “Melanotan I and II” products online, targeting consumers desiring to self-inject. It was apparent within the regulatory environment that these illegally synthesised products posed a danger to public safety.
The fulcrum of tension came unexpectedly in the middle of the European Medicines Agency (EMA) review of afamelanotide when 75 labs within the European Union were uncovered distributing “Melanotan I and II” as unapproved substances. As we tested these substances for high impurities, the chemical profile resembled peptide-like molecules, but not afamelanotide. However, as the market of self-tanning chemicals grew, regulatory bodies and national competent authorities grew more hostile to the pharmaceutical concept of photoprotection.
Now, a decade later as the dust has settled, we can state that at some point the suggestion was made that CLINUVEL was allegedly suspected of actually being one of the rogue laboratories distributing unregulated illegal substances. It was a sublime attempt – without grounds or evidence – and the doubts subsided as substantiated claims could not be maintained. It demonstrated that impressions and perceptions can actually rule a room of rational decision makers.
With scientific evidence, the consistency of public messaging and a history of integrity, our team managed to eventually conclude the case that CLINUVEL had no involvement or business in flogging illegal chemicals to consumers in EU, US, or Australian markets.
Adding to the prodigious chagrin, from 2005 to 2011 we witnessed parts of the medical community openly raise doubts whether afamelanotide should be used at all, and which side effects could be expected over the long run. During medical conferences, some scientists – without actually having evidence or real-life experience in using the drug product – would take to the microphone suggesting to the room that afamelanotide would not prove safe in the long run. Their main concern had been the drug’s ability to darken pre-existing sunspots (nevi), despite a growing body of scientific evidence pointing to the contrary effect: the hormone could actually photoprotect and guard the dermal and epidermal cells. Some reports would go as far as not being able to distinguish between rogue chemicals and the regulated drug afamelanotide, confusing the two.
This perpetual emphasis on safety allows us to translate melanocortins to other indications, other formulations and even OTC products.
All these global events surrounding melanocortins would naturally have an impact on the impression of scientists and reviewers at EMA, Australia’s TGA and the US FDA, who received affirmation of unfounded biases. However, our scientific and clinical data and arguments held at the end.
Now, a decade on, we take it upon ourselves to monitor patients life long. As time has passed, we have come to understand that binding to the MC1 and 4 receptors could theoretically be achieved by many agents, such as forskolin, prostaglandins and synthetic molecules. However, since all these substances differ in structure from the biological alpha-melanocortin stimulating hormone (α-MSH), long-term (>10 year) safety in large populations will need to be proven at the risk of starting non-physiological processes. CLINUVEL has gone down that long and dark lane, while it chose to work with an analogue of the natural α-MSH. Now, our teams have 70,000 exposure days to analyse and balance any future discussion, if any.
A Swiss financier asked me whether this process could have been completed earlier or faster, and the answer is negative. CLINUVEL had to overcome these legacy issues, proving afamelanotide’s safety beyond reasonable doubt to silence the most resistant regulatory reviewers and clinicians. As the contrast could not be bigger, our pharmacovigilance and quality teams are now applauded for their industrial approach; in pharmaceuticals, one can be confident without ever being able to rest on their laurels.
In a few days, we will have administered the 10,000th implant dose, as we enter the fifth consecutive year of “use under real world conditions”. This moment is one to cherish, given the background I provided you today. We had a plan, and we executed. This perpetual emphasis on safety allows us to translate melanocortins to other indications, other formulations and even OTC products.
In conclusion, the establishment of an integrated, in-house specialised team monitoring clinical safety, building systems and analysing live fed data daily had initially been borne out of defence; now, this approach has become an asset of the Company. The best way I can summarise our work is: “the safety efforts have costed a seven digit number, but preserved the Company’s nine figure valuation, without which patients would never have had the treatment”.
Us Update (Dr Linda Teng)
The US team continues to pave uncharted territory by identifying, evaluating, and training US EPP Specialty Centers as new clusters of EPP patients are engaged throughout the United States. With trained and accredited EPP Centers spread out nationwide, most EPP patients are receiving treatments with their local EPP Centers. In addition, the roll-out of US Centers has increased awareness of the EPP disease in both private and academic sectors of the US medical community. We hope that the ongoing awareness of EPP from US physicians and Centers will allow patients who were misdiagnosed or undiagnosed to have access to proper medical care and treatment. The expansion of US Centers has resulted in a continuous increase of EPP patients signing up for the EPP registry. The US team implements longitudinal care to new and current EPP patients by providing assistance and guidance to their local EPP Centers. In parallel, the US team maintains active interactions with EPP providers via telephone and email, and publishes quarterly clinical newsletters to US EPP Centers.
The US team is humbled to work with and for a wonderful group of US EPP patients
The COVID-19 pandemic has caused upheaval in the US healthcare industry. The lifting of restrictions and reopening the economy by individual States has resulted in a surge of backlog on medical appointments and procedures as hospitals rush to implement ramp-up strategies and address staffing shortages. This has also impacted the EPP patients’ treatment schedules. A number of EPP patients have reached out to the US team and expressed their challenges in not being able to schedule their first and/or subsequent visits with their EPP providers for the next 2-3 months. The close relationship with the EPP Centers enabled the US team to assist the EPP patients by bypassing the standard appointment system and successfully schedule timely first and/or treatment visits.
The US team has been the backbone in supporting and liaising between the US Centers and EPP patients on Center referrals, Prior Authorization (PA) submissions, benefits and claims management, and any administrative assistance the Centers may need to ensure the EPP patients receive timely treatment. The recent new HCPCS Level II J-code (J7352) for SCENESSE® as “Afamelanotide implant, 1 mg” has facilitated a smoother, predictable process in obtaining PA approvals from US insurers.
The newly revised procedural code CPT® 11981: “Insertion, drug delivery implant (e.g., bioresorbable, biodegradable, non-biodegradable)” was approved and published by the AMA CPT® Editorial panel in June. This will become effective as of 1 January 2022 for use in insurance claims with dates of service on or after 1 January 2022 and will further decrease administrative work and/or eliminate PA approval for the CPT® procedural code, simplify medical billing and facilitate reimbursement for EPP Centers.
The importance of knowing the long-term safety profile of novel drugs to treat lifelong diseases, such as EPP, is paramount. Fortunately for EPP patients, CLINUVEL’s perseverance and hard work have accumulated the long-term safety data on SCENESSE® over two decades. The feedback on the effect of the SCENESSE® treatment for EPP patients remains unchanged.
The US team is humbled to work with and for a wonderful group of US EPP patients and received several positive comments from the EPP community expressing their appreciation, gratitude, and care by the EPP providers and team on their professionalism and care. It is our pleasure to share some of the EPP patient comments (some information have been removed to protect privacy):
Miami EPP Center and team are professional, compassionate, and efficient. They take care of every detail and I very much appreciate their support. I am received every eight weeks with open arms!
– EPP patient from New Jersey
I am so unbelievably grateful to the Boston EPP Center and team for helping me and so many others gain access to treatment. They managed to get a large hospital, in the middle of a worldwide pandemic, to open its doors to those of us near and far that were struggling to gain access to treatment. Then they took on the insurance approval nightmare that most of us had all been going through and they turned it all around. One by one they started to get us approved and paved the way to treatment. I also gained a specialist finally local. This means the world to me. Knowing I have doctors that have a full understanding of EPP is priceless to me.
The impact the treatment has made in my life is unbelievable. I received my second implant at the beginning of June and did something never thought I would be able to do in my lifetime. I went on vacation to Myrtle Beach with my family. I did so well that on our last day we even rented a boat for a few hours. Having this experience with my family is everything to me. Being pain-free at the end of the day is like a gift. All the doctors involved with the EPP Center have helped change my entire world. I am so grateful and cannot express how thankful I am to them.
I would also like to express my gratitude to Clinuvel for bringing EPP medication to market. It certainly was a long road and although I am still adjusting to treatment, I can promise you I will never take for granted what it took to get here. I look forward to a future of pushing the limits with the treatment.
– EPP patient from Massachusetts
I would like to take this opportunity to thank the Detroit EPP Center for all their hard work in making it seem effortless in dealing with the insurance company and administering the treatment. Many thanks to you all for taking the time to make my life better. The treatment is not a cure, but it sure does make my life so much easier and more enjoyable. Also, I want to the staff at Detroit EPP Center for being so prompt and courteous during appointment days. You all make this process feel so effortless
– EPP patient from Michigan
Operations (Mr Lachlan Hay)
Changes are being implemented across the business to facilitate organic growth within a defined structure: four divisions operating globally to generate value for the Company, with a foundation in the Research, Development and Innovation (RDI) Centre at VALLAURIX in Singapore. This work continues as we evolve from offering a single commercial pharmaceutical product in targeted regions into a Group of businesses offering healthcare solutions to patients and other audiences worldwide.
The core division remains Pharmaceuticals – developing and delivering therapies for patients who lack alternatives. We recognise the potential of our technologies and knowhow, and have identified several patient groups with severe genetic, DNA repair, dermatological, and acute central nervous system (CNS) disorders and a high unmet medical need. We continue to evaluate the possibility of adding to these groups while progressing existing programs.
Under the purview of Drs Wright and Bilbao, we have seen the first patients treated in the CUV801 study, evaluating the potential of afamelanotide for the majority of arterial ischaemic stroke (AIS) patients who are ineligible for the standard of care (see News Communique III). Safety and clinical reports from the first three patients have given the specialist physicians and our team comfort to continue the study in these acutely affected patients. In parallel, work continues across a number of regions for the DNA Repair Program, progressing innovative protocols to confirm the role of afamelanotide in DNA Repair, with an initial focus on patients with xeroderma pigmentosum (XP). Restrictions to contain ongoing outbreaks of COVID-19 variants placed upon hospitals, clinics and decision makers have impacted these programs, but we still expect to provide clinical updates during the second half of 2021.
It is 12 months since the RDI Centre was opened in Singapore. The VALLAURIX team has been able to work throughout the pandemic under altered conditions (shifts and split teams) to progress the various projects. As a result, this facility continues to grow in capacity and output, focusing on both pharmaceutical and broader healthcare solutions products. Mindful of the interest in our technology from the industry around us, we keep progress in-house until the last minute to ensure the Company retains value from its IP and knowhow.
People are central to our values and our business. The global teams are growing, with headcount expected to increase further and new skills added, reflecting our ambition. It is equally satisfying to see individual talent grow within their roles. The end of financial year gives the opportunity to provide performance reviews to the global team, with a number making progress this year into more senior or managerial roles. We also evaluate incentives across the business to ensure our investments in talent provide long-term returns.
As we grow across the Divisions, adding new talent, it is our desire to maintain key approaches of our business while ensuring staff are able to succeed in their objectives. We often note that CLINUVEL operates differently to peers in our sector and is, at times, unconventional in its approach. This comes from working in an innovative and entrepreneurial space, understanding what is needed to successfully introduce novel technologies and some of the challenges – including those of illegal products outlined in the Communiqué above – which are only seen once inside the organisation and/or they form part of Company DNA. Thus, new staff joining the Group all experience a steep onboarding curve, and it is the role of the teams around them to ensure this journey can be undertaken successfully. It gives us confidence that our newer team members are stepping up to meet these challenges.
A second Operations Update Webinar is planned for late September to provide further detail on our progress.
CLINUVEL continues to focus on the management and growth of commercial operations in Europe and the USA, as well as the expansion of research and development activities.
Investor Relations (Mr Malcolm Bull)
Company News Flow
CLINUVEL continues to focus on the management and growth of commercial operations in Europe and the USA, as well as the expansion of research and development activities. Since News Communiqué III of 03 June 2021, we have:
- Presented to the Jefferies Healthcare Conference, see below;
- Treated three acute arterial ischaemic stroke (AIS) patients under the world-first clinical program announced in October 2020;
- Released an interview series (parts I-III) with Chair Willem Blijdorp which provide insights to his role and the performance and objectives of CLINUVEL;
- Announced positive cash flow results from commercial operations in the fourth quarter of the 2021 financial year, refer below for more details; and today,
- Released several announcements relating to the record financial results for FY2021.
CLINUVEL’s announcements are available on the CLINUVEL website and CLINUVELNews. More specifically, announcements to the Australian Securities Exchange are available on the investor pages of the CLINUVEL website.
Cash Receipts – Quarter Ended 30 June 2021
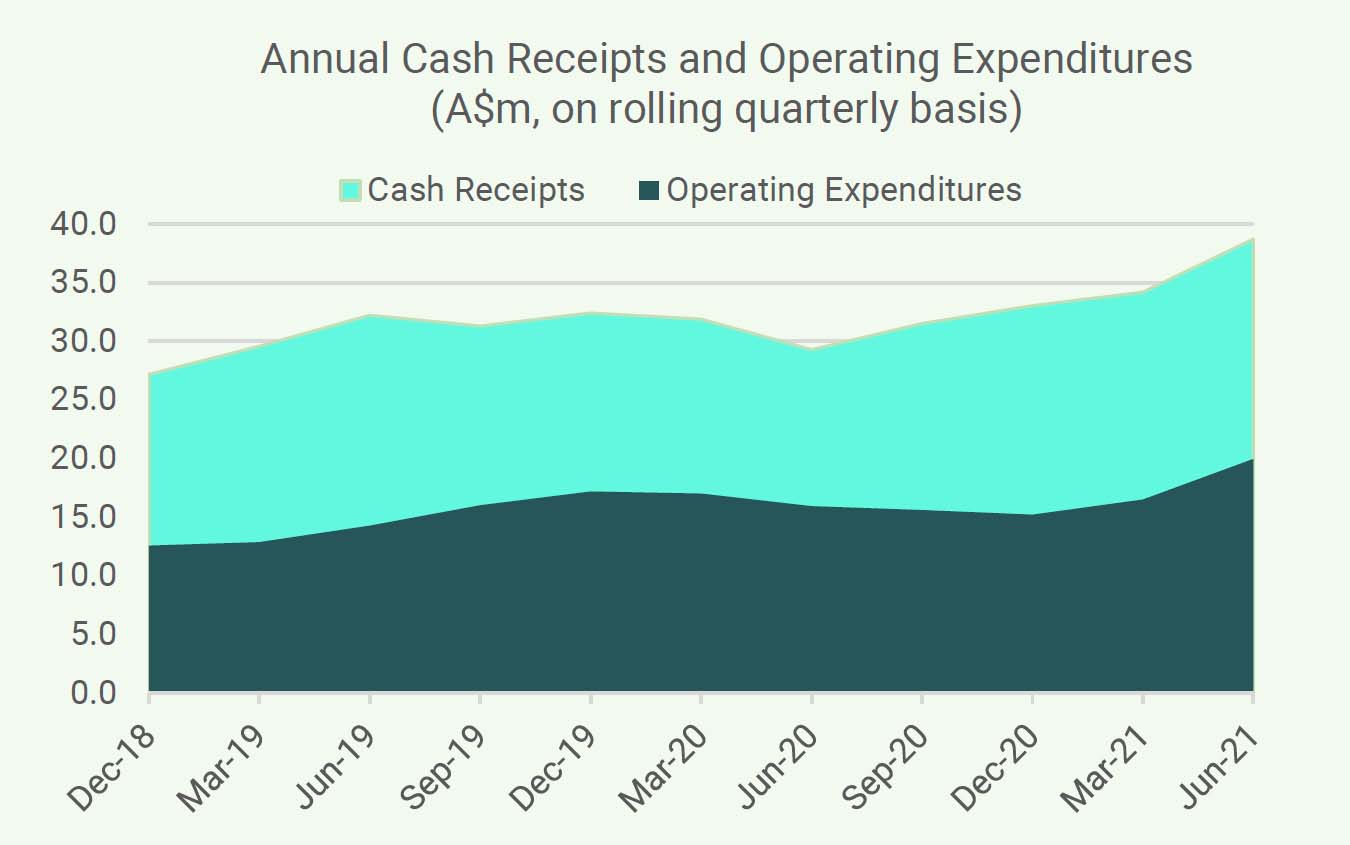
It is worth recapping that CLINUVEL generated positive cash outcomes over successive quarters of the 2021 financial year, culminating in a record result in the June quarter 2021. Cash receipts of A$14.918 million and net cash inflow (after operating expenditures) of A$8.102 million was achieved in the June quarter 2021. On a financial year basis, cash receipts were A$38.723 million, up 32% on the same period of 2020.
The trend in annual cash receipts on a quarterly rolling basis (see the graph below) reflects the near normalisation of treatment of SCENESSE® for EPP patients in Europe after an initial adverse coronavirus impact in the June quarter of 2020, and the rising contribution from the new commercial operations in the USA. The number of US Specialty Centers trained and accredited by CLINUVEL to administer SCENESSE® has increased to over 40 and there are more than 60 private insurers involved in the reimbursement of the cost of treatment of SCENESSE®. Expenditures have increased on an annual basis and continue to be well managed to support the growth and expansion of the business.
Recent Investor Meetings
Following the release of the June quarter 2021 cash results, Chief Financial Officer, Darren Keamy, and I met with investors, both institutional and private. Investor calls were hosted by Wilsons and Moelis involving over 20 institutions. Interest in the Company’s progress was keen, with a focus by some on the upcoming results for the financial year to June 2021. We were asked what the cash receipts outcome means for the likely revenue and profit outcome for the financial year. As we don’t provide forward guidance, we commented that:
- the bulk of the growth in cash receipts has been driven by US commercial operations;
- the cash receipts cycle in the US is longer than Europe, but sales revenue is booked in both geographies upon receipt of an order; and
- the data for cash receipts and expenditures in the March and June quarters of 2021 follows the strong revenue and profit outcome in the first half to December 2020, compared to the corresponding prior period.
We impressed on meeting participants that the record cash receipts outcome in the June quarter 2021 was an outstanding achievement in the context of the Company’s long-term strategy and the integrated business model we have employed. In addition, the impact of the COVID-19 pandemic has been more severe in CLINUVEL’s primary markets of Europe and the USA. Thus, to normalise growth in Europe and achieve progress in the USA, ahead of plan in this difficult operating environment, reflects the dedication and determination of the CLINUVEL team, the commitment of the healthcare sector to see patients treated and indeed, patient demand to be treated.
Communication of CLINUVEL’s Results
We now know the record results for FY2021 which crown five years of commercial operations. The activities of investor relations today, and over the ensuing week, are focussed on communicating the financial results to stakeholders. The schedule of communications involves senior executives of CLINUVEL in webinar briefings (refer below), one on one investor meetings, meetings with existing and potential investors hosted by analysts and investment banks, and a raft of media interviews, supported by social media posts. I look forward to briefing you on these activities in the next communiqué.
CLINUVEL will host an investor and analyst webinar at 18:00 AEST today to discuss the financial results for FY2021. Participants can register using the link below:
Investor Zoom Webinar 18:00-18:30 AEST (10:00-10:30 CEST) today (26 August)
To participate, please register using this link:
https://zoom.us/webinar/register/WN_0jevdk07TFaeOAgbsW1ruw
Questions may be tabled as you register or during the webinar
Conference Events
Over the course of the 2021 financial year, we participated in nine key conferences and presented to six to communicate our story and meet investors. We participated in the Jefferies Healthcare Conference on 01-04 June. Selected meetings with interested investors were held and we received positive comment on Dr Wolgen’s fireside chat with Dr David Stanton of Jefferies.
Attention is drawn to the key events for the remainder of the calendar year, noting there are three conferences at which CLINUVEL is confirmed to present.
Coming Updates
In terms of updates to be provided in September, you can expect first, Strategic Update III which will review CLINUVEL’s pipeline and update you on the progress of our clinical programs. Then, towards the end of the month, we will hold Operations Update Webinar II.
| Month | Key Events |
|---|---|
| January | JP Morgan Healthcare Conference, San Francisco HC Wainwright Bioconnect Investment Conference |
| March | Daiwa Investment Conference, Tokyo |
| May | UBS Global Healthcare Conference |
| June | Jefferies Healthcare Conference |
| September | HC Wainwright Global Investment Conference |
| October | Morgans 6th Annual Value in the Vines Investor Conference Citi’s 13th Annual Australia & New Zealand Investment Conference (tbc) |
| November | Jefferies London Healthcare Conference |
Conclusion
It has been an eventful and successful year for increased patient treatment and the delivery of record financial results in a challenging operating environment. We can all be proud of the results and the position we are in to progress the growth and expansion strategy of the Company.
To all of you, I express our appreciation for your continued support.
Philippe Wolgen
1 The SCENESSE® summary of product characteristics is available on CLINUVEL’s website.
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD.
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance, or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products, the COVID-19 pandemic affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical products; competition for our products, especially SCENESSE® (afamelanotide 16mg); our ability to achieve expected safety and efficacy results through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, China and Japan of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE® which may lead to it being unable to supply its commercial markets and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; any failure to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2021 Preliminary Final Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on the forecasts and estimates is available on request. Past performance is not an indicator of future performance.
Contact
Level 11, 535 Bourke St
Melbourne, 3000 Vic,
Australia
+61 3 9660 4900
+61 3 9660 4909