CLINUVEL Communiqué V
| Melbourne, Australia, 22 November 2021 | ASX: XETRA-DAX: NASDAQ INTERNATIONAL DESIGNATION: |
CUV UR9 CLVLY |
Dear Shareholders, Friends
Introduction
As we head into the northern hemisphere winter, our teams have already set much of the course for 2022, with key commercial and clinical milestones established.
Despite the ongoing restrictions of the pandemic and new waves of infections across much of Europe, there is some optimism that – with vaccines – we have adjusted to the realities of COVID-19 and can begin to operate according to a “new normal”. As part of this, we are already seeing our clinical, commercial and investor teams being able to facilitate more of their work in the physical, rather than virtual, world – a trend we all hope will continue. Hospitals and healthcare systems have equally adapted and, while this optimism should not be cause for complacency, we hope to see a more predictable year of clinical and commercial operations in 2022.
Understanding Treatment for Erythropoietic Protoporphyria (EPP)
Since 2006 the CLINUVEL team has focused on the development of the first ever treatment for patients with the rare metabolic disorder erythropoietic protoporphyria, or EPP. Perhaps unsurprising for those who work with rare diseases, but less recognised by many of our readers, is that our team is constantly learning new facets about the unique nature and impact of this disorder upon patients and those who care for them.
Like many rare diseases, EPP is poorly characterised. This does not mean the disease is not understood mechanistically,1 but rather that the overall patient experience has not been well expressed or quantified, despite being first described in the medical literature in 1961. Put simply, EPP is a lifelong, inherited genetic disorder of haem biosynthesis that leads to an absolute intolerance to light, particularly in the visible (blue) and near-visible (ultraviolet, UV) spectrum. The patient experience, however, is far from simple.
Most EPP patients report a level of light intolerance – one which varies according to previous exposure, general health and a range of suspected contributing factors – which translates to an inability to expose to light (including sunlight) for more than a brief period of time, measured in seconds or minutes. After brief exposure to light, some (but not all) EPP patients will experience a prodromal symptom, a warning sign that further exposure will result in a debilitating cutaneous reaction, phototoxicity.
In the event of overexposure (again, a matter of minutes), EPP patients will experience the full extent of a phototoxic reaction, a deep burning sensation as the capillaries in the deeper layers of the skin are damaged or destroyed. This will manifest in time with visible symptoms, often first appearing as only mild swelling or erythema, despite the patient being incapacitated with a sensation likened to neuropathic pain. Later, deeper wounds and scarring will occur. Further stimuli, such as heat variation, pressure, and further exposure to light, exacerbate the reaction and patients will often be unable to sleep through the night. Analgesics – of any kind, including morphine – have no effect on the reaction. Parents are unable to console children with EPP with physical contact as it exacerbates the reaction. Adult patients – aware of their affliction even if undiagnosed – will fundamentally change their lives to avoid exposure.
EPP has a marked impact upon patients’ mental health. Understanding the consequences of overexposure, patients become hyper-aware of light in any environment, anxious that they may be unable to avoid a phototoxic reaction. In parallel, many report a “sixth sense”, an undefined relationship with their conditions such that they are acutely aware of the amount of exposure necessary to cause the onset of a reaction. (This intuition is often linked with the “priming” phenomenon seen in EPP, where cumulative brief light exposure over several days is sufficient to lead to a phototoxic reaction). A patient once summarised this experience to me as having a constant low level “hum” in their life, akin to interference noise on a radio frequency: a relentless reminder that a phototoxic reaction may be only a few moments away. The patient – who was also medically qualified – further noted that the hum was a tireless distraction, a perpetual burden of disease that made it impossible to fully focus.
Treatment with afamelanotide had, for this patient, eliminated the hum. It provided a freedom not only from phototoxicity, but most of all from the fear and anxiety that accompanied a constant awareness of light exposure. It provided a new and much desired “normal”.
Anecdotes like these not only provide our team with a strong motivation to continue our work, but they spark a curiosity to better understand the diseases that we seek to address. Throughout our EPP program we have worked to collect data to understand the disease and seek to improve outcomes for patients. In Europe this work has focused for the last five years on the establishment of the European EPP Disease Registry (EEDR), the largest registry of its kind for EPP and likely the best research resource that has ever existed for the disease.
Each year we capture thousands of pseudonymised data points from the European EPP community, tracking their health and wellbeing, and the long-term effects of treatment. While focused primarily on safety (the EEDR is part of the ongoing European post-authorisation safety studies for SCENESSE®), the registry also captures effectiveness outcomes, data indicating the ongoing therapeutic benefit of treatment. Collectively this real-world evidence provides us (and authorities) with comfort of the safety profile of SCENESSE® in EPP as well as ongoing indication of clinical benefit.
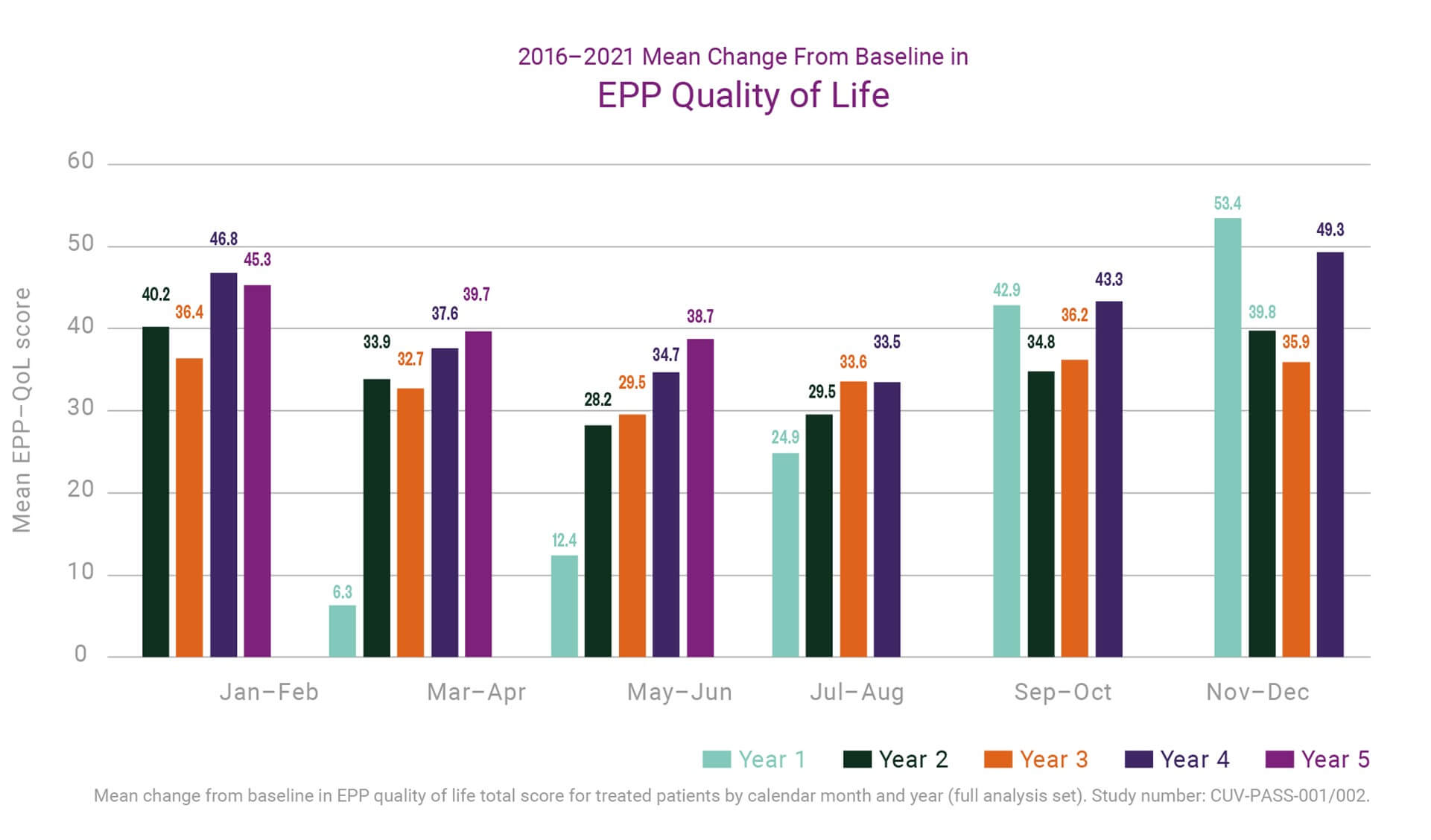
Each year Dr Bilbao’s team conducts analyses of data from the EEDR to report to authorities, a task which is ongoing at present for 2021. The most recent report, capturing data up to a “lock” point of 22 June 2020, provided some new insights to the team on the longer-term effects of treatment which are relevant to share here.
By June 2020 a small number of EPP patients enrolled in the EEDR had been on treatment for over ten years across clinical trials, compassionate and special access programs, and the EU marketing authorisation. Many more had received five or more years of treatment and most at least two years. Not only was the safety profile of the product maintained in these patients, but trends had begun to emerge on the cumulative impact of treatment. Across five years we have seen a consistent improvement in patient quality of life (QoL; measured by the disease specific EPP-QoL tool) compared to baseline. More interestingly, the average improvement seen during the summer months (when patients are at greatest risk of phototoxicity from increased environmental exposure) increased year on year from the first to fifth year of treatment (see the chart below). This suggests an even greater benefit from long-term treatment.
It provided a freedom not only from phototoxicity, but most of all from the fear and anxiety that accompanied a constant awareness of light exposure. It provided a new “normal”.
In parallel, the data up to June 2020 suggested that patients experienced fewer episodes of phototoxicity in springtime clinical visits for years 2 through 5 of treatment compared to their first year, and that reactions overall were less severe. Finally, composite measures used to evaluate light exposure and phototoxicity indicate that patients’ overall light tolerance is increased in each additional year of treatment.
The data are compelling and will be further evaluated before any additional claims will be made. Nevertheless, these help guide our thinking, discussions, and approaches to further development of treatments for EPP, particularly with a view to facilitating a safe and effective treatment for paediatric patients.
Since the first Phase II study of afamelanotide in EPP (CUV010) was published in the New England Journal of Medicine in January 2009 there have been marked advances in the understanding of EPP, its impacts upon patients’ lives and the role of the first ever photoprotective therapy. As we progress our program, CLINUVEL’s team is keen to ensure we can continue sharing our knowledge and learnings for the benefit of patients.
Post-Authorisation Supply and Demand
CLINUVEL is not immune to global trends and, like many businesses, 2021 has seen the Company’s supply chain tested. In particular, the lack of aviation freight capacity (to ship under cold storage conditions) and backlogs at our supply partners have pushed our team to pursue an exhaustive approach to ensure supply. Our focus is always safety first, ensuring that any measures we implement do not place any individual at risk, in this instance the patients we seek to help.
Our approaches would not be possible, however, without regulatory and clinical support. In a heavily regulated environment, those who oversee the use of a product and maintain personal responsibility for patient care are equally involved in ensuring risks can be mitigated and safety maintained. The continued support from both regulators and expert centre staff (pharmacy and clinical teams), reflects the ongoing need to treat EPP patients and understanding of the value of treatment access. In parallel, we are grateful to our European and US teams responsible for product distribution, quality assurance, and regulatory interactions for their ongoing commitment. Your late nights, delayed holidays, and overall determination to help patients is well recognised.
Despite the shortening of daylight as winter draws closer in the northern hemisphere, the demand for EPP treatment appears to be consistent and year-round for many patients. As a result, we are adapting our supply chain and evaluating how to ensure its robustness longer-term to meet the next challenges, particularly as more and more patients pursue treatment.
CLINUVEL is not immune to global trends and, like many businesses, 2021 has seen the Company’s supply chain tested.
Expanding CLINUVEL’s Specialty Melanocortin Portfolio: NEURACTHEL®
Over two decades, CLINUVEL’s team have developed unparalleled expertise in the use of melanocortins, a specific group of bioactive hormones and their analogues. These peptides (short chain molecules comprising amino acids) bind to the five melanocortin receptors (MC1R through MC5R) on cells throughout the body, exerting effects, including on pigmentation, inflammation, energy homeostasis, appetite, and sexual function.
CLINUVEL’s first commercially approved product SCENESSE® is an injectable, subcutaneous, controlled-release implant formulation of the melanocortin afamelanotide, an analogue of the naturally occurring alpha-melanocyte stimulating hormone. Afamelanotide is well understood to bind primarily to the MC1R and MC4R and exert its effects on cells throughout the body. It is from this understanding – and the understanding of appropriate dosing through the controlled-release formulation – that CLINUVEL has been able to successfully commercialise the SCENESSE® implant for EPP and develop novel liquid formulations for clinical use as PRÉNUMBRA®.
In addition to afamelanotide, CLINUVEL has developed a portfolio of novel proprietary melanocortins which are being progressed in pre-clinical stages. CUV9900 and VLRX001 are both being assessed for their potential to assist specific target groups.
As part of CLINUVEL’s development work with melanocortins, the Company has established a unique expertise in formulatory work, with the Research, Development and Innovation Centre at VALLAURIX Singapore arriving at a range of viable dosing platforms for next generation products. These platforms allow for controlled dosing of peptide drugs, leveraging key learnings from the development of SCENESSE® and PRÉNUMBRA®.
Central to CLINUVEL’s approach has always been the application of our expertise and technology for patients who lack therapeutic alternatives. One sees this in our delivering of a first-ever treatment for EPP patients, as well as our clinical focus on xeroderma pigmentosum (XP; no treatment), vitiligo (no recognised standard of care) and ischaemic stroke (most patients ineligible for standard of care). It is thus within the remit of our Group to seek to deploy our expertise where opportunities arise.
Adrenocorticotropic hormone (ACTH) is one such opportunity that has attracted our attention during the past years. As announced in early November, CLINUVEL is developing novel formulations of ACTH as NEURACTHEL®, with both instant and modified-release doses, having secured supply of the drug substance to a current Good Manufacturing Practices (cGMP) standard. Unlike afamelanotide – where CLINUVEL has had to develop the environment to accept a novel melanocortin and establish the safety profile – ACTH is well recognised by the medical and regulatory communities. There is already a global demand for the product, estimated to reach US$1.91 billion by 2031, and disruptions to supply of existing formulations for patients with a range of disorders. Where we see greatest value, however, is for those patients who may be assisted by NEURACTHEL® yet remain neglected by medicine, a genuine unmet medical need. As our programs progress, we will share more on the intended pathways for NEURACTHEL®.
CLINUVEL is developing novel formulations of ACTH as NEURACTHEL®, with both instant and modified-release doses.
Investor Relations (Mr Malcolm Bull)
Recent Events
CLINUVEL has recently engaged in multiple conferences attended by institutional investors showing interest in our EPP, DNA Repair and AIS (stroke) programs and the addition of our over the counter (OTC) product lines. We have also been active in the media and through webinars to communicate CLINUVEL’s story.
A summary of the recent presentations and interviews given by CLINUVEL’s management is provided below:
- CLINUVEL Annual Results Webinar, 26 August;
- Ausbiz interview, 26 August;
- Youtube interview with M. Rohde, 28 August;
- TeamInvest Webinar, 30 August;
- HC Wainwright Global Healthcare Conference, 13 September;
- Goldman Sachs Healthcare Day, 06 October;
- Operations Update Webcast II, 07 October;
- Morgans Scone, Value in the Vines Investor Conference, 22 October;
- CLINUVEL Investor Webinar, 26 October;
- Nasdaq “TradeTalks” interview, 26 October;
- Youtube interview with M. Rohde, 7 November;
- Strategic Update III, 08 November;
- The Chair’s Address and Managing Director’s Presentation to the 2021 AGM,
10 November; and - Jefferies London Healthcare Conference, 18 November.
At the Value in the Vines conference, an extensive and engaging discussion was held between Morgans Scone principals, Sam Paradice and Shaun Trewin, and our Managing Director, Dr Philippe Wolgen. We trust our readers will gain new insights into the foundation and direction of CLINUVEL from the hour-long fireside session.
We would also like to correct any misunderstanding from the title of CLINUVEL’s October interview with Nasdaq TradeTalks, “Seeking Access to Nasdaq to Raise Capital”. CLINUVEL’s assertion was that many early-stage companies seek funding through a Nasdaq listing to finance their operations and that CLINUVEL’s position is different to these companies. The last five years of commercial operations have enabled sufficient cash reserves to be built to self-finance CLINUVEL’s planned organic growth and expansion. The argument made during the interview was that CLINUVEL is operating “from a position of strength” and this differentiates us from many peers.
Annual Report 2021
It is timely to report in this communiqué that we received considerable positive feedback on the 2021 Annual Report, released on 08 October. The content was found to be informative, supported by effective design and graphics. Others commented that the theme of the ‘opening of the door’ to CLINUVEL’s growth and expansion illustrated the Company’s journey to date and provided the context for its strategic initiatives. We feel all stakeholders can rightly feel proud of their support of the Company and its achievements, as reflected in the Annual Report 2021. The 2021 Digital Annual Report is available on the Company’s website.
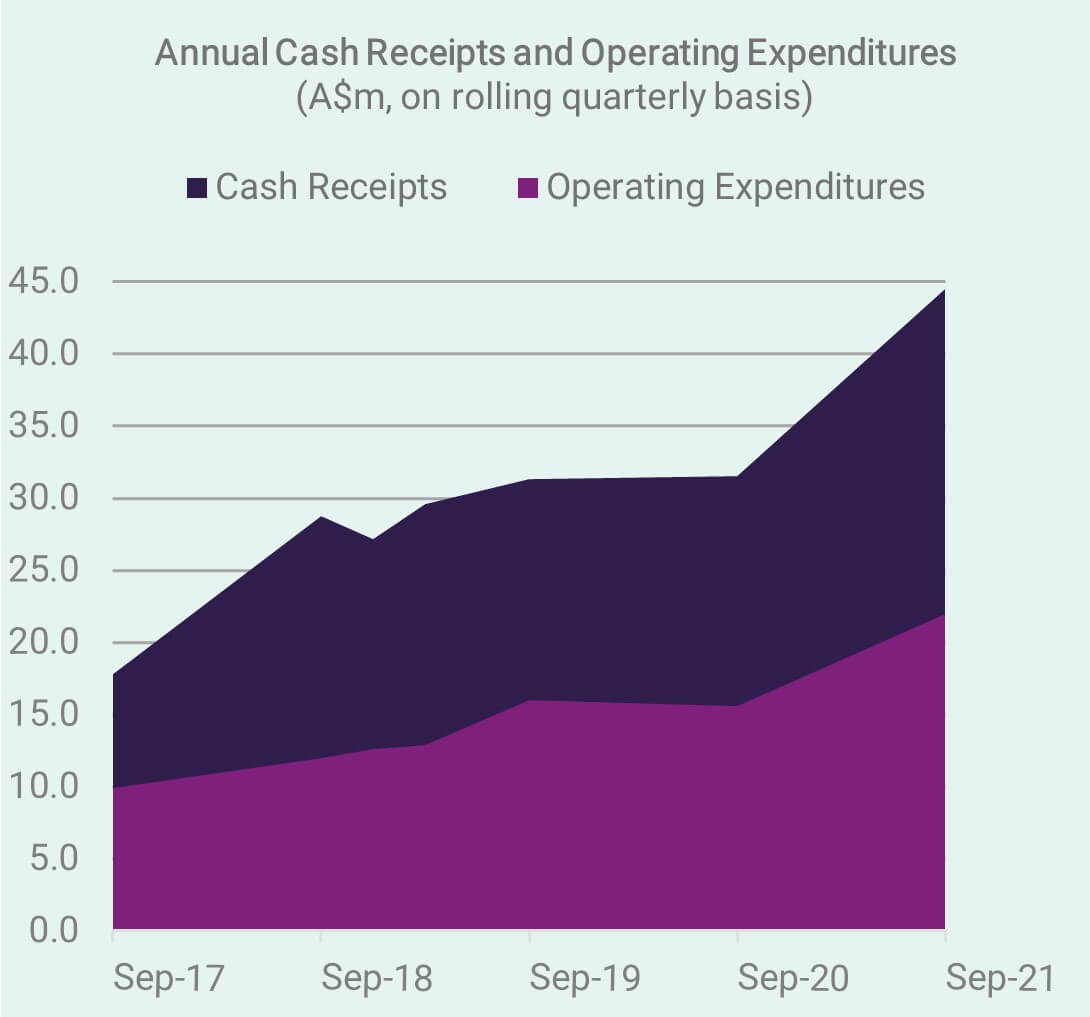
Cash Receipts – September Quarter 2021
We look back at another excellent quarter (ending 30 September), with the trend of increasing cash receipts continuing to lay the foundations for growth, reinvestment in the business, and maintain the buffer necessary to manage the next cyclical corrections we may see in equity markets. Investors are encouraged to review the latest Appendix 4C & Activity Report.
As a profitable enterprise, generating positive cash flow from commercial operations, CLINUVEL is not required by the Australian Securities Exchange to issue quarterly cash receipts and activity reports. However, we will continue to report quarterly as a courtsey to shareholders. Without this, the formal announcement of financial related results would be limited to the half year and full year financial accounts.
AGM 2021
The key event of a company’s investor relations calendar is without doubt, the Annual General Meeting of Shareholders (AGM). CLINUVEL held its second virtual AGM on 10 November. Whilst we much prefer to have an in-person AGM and hope this can resume in 2022, we utilised the technology available to engage shareholders around the world for the second consecutive year, due to the coronavirus pandemic. At the Meeting, the Chair and Managing Director delivered their addresses and shareholders voted on key resolutions and utilised the opportunity to ask questions. Overall, the great majority of shareholders expressed the wish not to see any disruption to the Company’s course.
Following feedback received from shareholders on CLINUVEL’s executive remuneration at the 2020 AGM, we consulted extensively with external advisors, proxy advisors and of course, shareholders. Our response in Remuneration Report 2021 was to provide a more detailed explanation of our approach to remuneration and enhance disclosure on the remuneration structure and conditions that need to be achieved for an award of STI and LTI. The Report also contained an updated benchmarking analysis which showed the MD’s total remuneration is below the median of a group of Australian and US listed companies with comparable characteristics to CLINUVEL.
The more detailed explanation of remuneration, enhanced disclosure and updated benchmarking analysis, was recognised by the two leading independent proxy advisors (CGI Glass Lewis and ISS) by supporting voting on the resolutions of the AGM in line with the recommendations of the Board. We are pleased a large majority of shareholders made an expansive assessment of these important remuneration matters, and recognised CLINUVEL’s leadership amongst peers in terms of total shareholder returns – specifically, a 591% increase in the CUV share price over 5 years with four consecutive annual dividends. As a result, all resolutions of the Meeting were supported in line with the recommendations of the Directors. As no ‘strike’ was recorded (with less than 25% of shares voted Against the Remuneration Report), the conditional ‘spill’ resolution did not need to be considered. Two Non-Executive Directors, Dr Agersborg and Mrs Smith, were re-elected to continue their positive guidance and overview of the Company as key members of the CLINUVEL Board.
CLINUVEL is buoyed by an increase in both member participation and the number of shares voted at this year’s AGM. This is the result of a concerted effort over the past year to communicate more widely and differently to build relationships with shareholders around the world. This is evident in more frequent investor webinars, operations update webcasts and strategic updates, media interviews, regular Chair Letters, and news and scientific communiqués. As the Chair mentioned in his letter to shareholders in the 2021 Annual Report, “we also made an effort to connect with shareholders who hold their shares through one or more custodians; time will tell how effective this process has been”. As difficult as it can be to communicate with shareholders through multiple custodians, a difference was made and shareholder participation in CLINUVEL is all the better for it. We appreciate the support of all shareholders, particularly those who voted at the AGM this year to support the Company, and will continue to engage you with positivity and by maintaining our leadership in the issuance of frequent updates on our progress.
On a sadder note, we have also witnessed the passing of David Blake, a true leader in the Asia-Pacific life-sciences investor community and co-founder of Bioshares. Over the years his insights have benefitted many of us, and we take this opportunity to share an obituary written by CLINUVEL’s CEO Philippe Wolgen.
The final point in this communiqué from Investor Relations, is to note all of CLINUVEL’s announcements are available on the CLINUVEL and CLINUVELNews websites. More specifically, announcements to the Australian Securities Exchange are available on the investor pages of the CLINUVEL website.
The DNA Repair Program seeks to confirm the role of afamelanotide in rejuvenating DNA… …there are, potentially, two billion individuals worldwide who are deficient in DNA repair mechanisms.
Clinical Development Programs
CLINUVEL’s ongoing clinical programs seek to prove the safety and clinical benefit of melanocortins for a range of patient groups who lack alternatives. Each program is currently investigating very different mechanisms of the potential of melanocortin therapy which may benefit patients with a range of chronic and acute conditions. Before pursuing a clinical program, the Company sets clear objectives to ensure the considerable investment necessary can be justified, and all risks are identified and managed to the standards expected of our team.
The DNA Repair Program seeks to confirm the role of afamelanotide in rejuvenating DNA which has been damaged by exposure to ultraviolet (UV) and visible light. While there are, potentially, two billion individuals worldwide who are deficient in DNA repair mechanisms, one group of patients are most acutely affected: those living with xeroderma pigmentosum (XP). The exact global prevalence of the eight XP complementation groups is unknown, but expected to range from 1 patient in every 22,000 to 1 million inhabitants. Akin to many genetic disorders, one sees regions of much higher prevalence where a particular genetic defect is more prominent and has been carried through generations of families.
We have previously discussed, at length, the challenges posed to conduct a clinical program involving XP patients who, due to their genetic disorder, have a shortened life expectancy and are rightly protected by the physicians who provide clinical care, and the regulatory and ethics bodies responsible for approving clinical studies. It was thus encouraging for our team to receive approval to commence the first study focusing on treating XP-C patients (CUV156). With the first patients enrolled, the treatment has been well tolerated to date and no unexpected adverse reactions have been reported.
Our recent investor webinar addressed several questions received on the vitiligo program and gave an update on the breakthroughs achieved to progress to our next study, CUV104. Vitiligo, a depigmentation disorder, has no approved therapy, with current standard treatments offering limited clinical benefit and often requiring many months of repetitive therapy, placing a further burden on patients and their families. Patients are highly stigmatised and report a loss of identity, needing or choosing to withdraw from their society and environment as a result of their condition and its visible impact. CLINUVEL’s approach with afamelanotide has evolved since the initial studies, with a targeted population – those with darker skin types (Fitzpatrick IV-VI) who respond best to the systemic therapy as a repigmentation agent – clearly defined and a protocol for the CUV104 study now agreed with global experts. The Company now awaits clearance from the US Food and Drug Administration (FDA) to commence CUV104 in the USA.
Our recent investor webinar addressed several questions received on the vitiligo program and gave an update on the breakthroughs achieved to progress to our next study, CUV104.
Conclusion
Over the coming months we expect to be able to share further updates on our programs, including news on the new indication for afamelanotide and progress from our VALLAURIX Research, Development and Innovation Centre. We look forward to sharing this with you.
Lachlan Hay, Director of Global Operations
1One must, however, acknowledge that much about EPP remains poorly defined, with issues such as liver impairment and the effects of pregnancy on phototoxicity areas of ongoing research.
– End –
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD.
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products; the COVID-19 pandemic and/or other world, regional or national events affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical products; competition for our products, especially SCENESSE® (afamelanotide 16mg), PRÉNUMBRA® or NEURACTHEL®; our ability to achieve expected safety and efficacy results in a timely manner through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, Israel, China and Japan of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE®, PRÉNUMBRA® or NEURACTHEL® which may lead to it being unable to supply its commercial markets and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology and consumer based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; our ability to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2021 Annual Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on preliminary and uncertain forecasts and estimates is available on request, whereby it is stated that past performance is not an indicator of future performance.
Contact
Level 11, 535 Bourke St
Melbourne, 3000 Vic,
Australia
+61 3 9660 4900
+61 3 9660 4909