Media Release – New drug to repair damaged skin and reduce skin cancer risk
A leading Australian pharmaceutical company is testing whether a treatment can help repair skin damage caused by exposure to ultraviolet (UV) and visible light. If successful the drug, SCENESSE® (afamelanotide 16mg), would slow the rates of photoaging and reduce the risk of skin cancer.
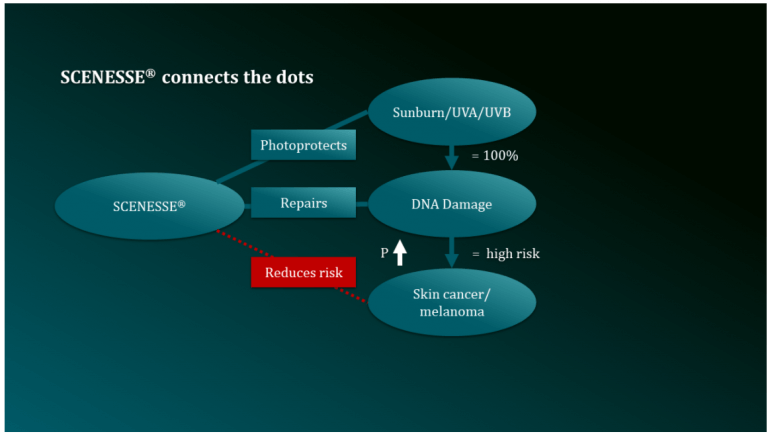
SCENESSE® has shown positive results in preventing and repairing DNA damage caused by the sun, which leads to premature ageing through collagen and elastin reduction in skin while UV exposure increases the risk of development of skin cancers. The Australian company, CLINUVEL, is running clinical trials to confirm the ability of SCENESSE® to repair the DNA in skin cells, focusing initially on patients who are at a 10,000-fold risk of skin cancer due to a rare disease known as xeroderma pigmentosum (XP). XP is the most extreme form of intolerance to sunlight when patients develop skin cancers at a high rate.
“Light and UV radiation are constantly attacking our skin cells, causing damage to our DNA,” Dr Pilar Bilbao, CLINUVEL’s Clinical Operations Manager said. “The body can repair most of this damage, but over time or when we have too much UV exposure, our natural defence mechanisms fall short. When this happens the damage accumulates, leading to visible photoaging and possibly skin cancer in fair skinned people.
“Much research has shown that agents like SCENESSE® have the ability to aid the DNA repair process. We are testing this for the first time in individuals with XP – patients who are unable to repair skin damage on their own – and will rapidly expand our research as results allow.
“We look forward to confirming what we have seen in earlier clinical research: we have the technology and knowhow to repair DNA damage in humans, thus potentially reducing the risk of skin cancer,” Dr Bilbao said.
Skin damage from UV and visible light – known as photodamage – is most commonly the result of exposure to sunlight, but can also result from overexposure to artificial light and radiation, including from phones and computer screens. When light penetrates the skin, it damages the DNA within skin cells.
CLINUVEL is developing a range of products based on its very targeted research with SCENESSE®, including topical formulations of its proprietary drugs and over-the-counter products, and has recently opened a new Research and Development Centre in Singapore to accelerate this work. First results from the use of SCENESSE® in XP patients are expected in 2021. The scientific data from SCENESSE® has led to further products for general use.
1 SCENESSE® (afamelanotide 16mg) is approved in the European Union as an orphan medicinal product for the prevention of phototoxicity in adult patients with erythropoietic protoporphyria (EPP). SCENESSE® is approved in the USA to increase “painfree” light exposure in adult EPP patients with a history of phototoxicity. Information on the product can be found on CLINUVEL’s website at www.clinuvel.com.