Media Release (ASX) – CLINUVEL progresses innovative DNA Repair Program
Drug tested to protect skin and regenerate DNA, firstly in XP patients at 10,000-fold skin cancer risk
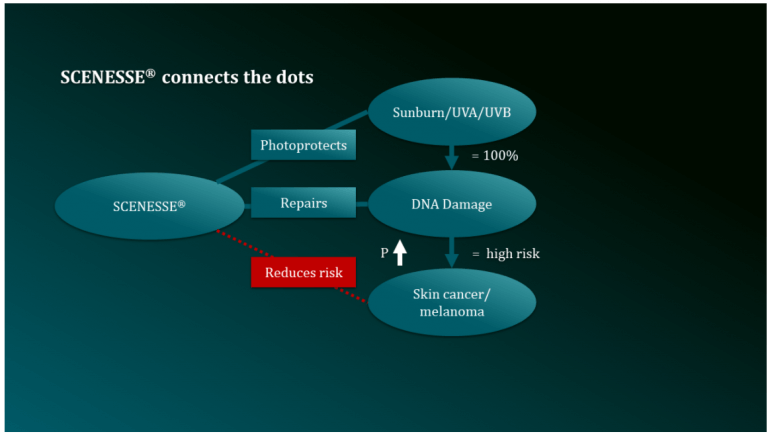
CLINUVEL PHARMACEUTICALS LTD today announced the progression of its drug SCENESSE®(afamelanotide 16mg) to treat the disease xeroderma pigmentosum (XP). The aim of the development program is to confirm the drug’s ability to regenerate DNA of skin exposed to ultraviolet (UV) damage.1
Skin incurs DNA damage following UV exposure. Non-ionising light penetrates the nucleus of skin cells and causes distortions to the DNA helix known as photoproducts. If left unrepaired, these chemical changes to DNA may replicate as mutations, leading to irreversible damage (photoaging) and further progress to skin cancer, including melanoma.
Human biology contains complex mechanisms to protect itself from UV damage and restore cellular DNA to its original state. People of fair-skinned complexion are known to have defects in these UV protective and DNA regenerative processes, increasing their long-term risk of skin cancer.
Having commercialised SCENESSE® in Europe and the USA for the rare genetic disorder porphyria (EPP), CLINUVEL is expanding its clinical research, aiming to confirm how intervention with the drug enhances elimination of photoproducts and regeneration of DNA.
“In SCENESSE® we have a hormone acting on various organs and receptors, but most of all known to protect human DNA,” CLINUVEL’s Chief Scientific Officer, Dr Dennis Wright said. “Our task is to confirm how DNA regeneration occurs within genetically affected patients and healthy subjects.”
Following decades of clinical experience with SCENESSE®, CLINUVEL is now accelerating a comprehensive DNA Repair Program by first treating patients with the rare genetic disorder xeroderma pigmentosum (XP) who have the most extreme deficiencies in their DNA repair processes, leading to a 10,000-fold increase in their risk of skin cancer.
“Tragically, XP provides an extreme model of what happens to our skin if UV-induced damage is left unrepaired,” Dr Wright said. “From a young age, XP patients are incapable of responding to DNA damage caused by UV exposure and experience disfiguring and aggressive skin cancers.
“After two decades of clinical research, I’m delighted that our team can now focus on the XP patients who are severely affected by UV radiation leaving them a short life expectancy. We will facilitate treatment for the first patient in the next few weeks,” Dr Wright said.
The first clinical results from the DNA repair program are expected to be reported in 2021.
1 SCENESSE® (afamelanotide 16mg) is approved in the European Union as an orphan medicinal product for the prevention of phototoxicity in adult patients with erythropoietic protoporphyria (EPP). SCENESSE® is approved in the USA to increase “painfree” light exposure in adult EPP patients with a history of phototoxicity. Information on the product can be found on CLINUVEL’s website at www.clinuvel.com.