Media Release – First patient dosed in SCENESSE® DNA Repair Program
Xeroderma pigmentosum (XP) patient receives SCENESSE® treatment
In the search for a preventative treatment of skin cancers, including melanoma, it is imperative to understand and treat DNA damage caused by ultraviolet (UV) radiation. Following the treatment of a patient suffering from xeroderma pigmentosum (XP), a disease characterised by an inborn insufficiency to repair DNA damaged by sun exposure, Australian based CLINUVEL PHARMACEUTICALS is the first company worldwide to use a systemic therapy to repair DNA.
SCENESSE® IN XERODERMA PIGMENTOSUM
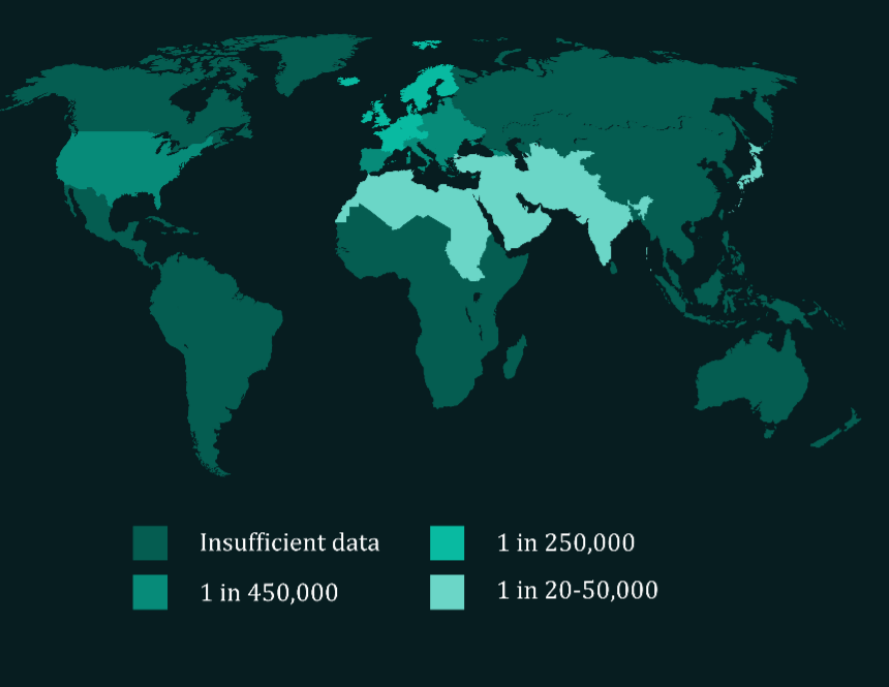
XP is a genetic disease which has served as a human model for studying the insufficiency of human DNA repair. Patients develop frequent skin cancers from an early age – most experience their first malignancy before adolescence – and must avoid all forms of UV exposure. The disease has a high mortality rate, with a median life expectancy of thirty years. XP treatment is limited to management of symptoms, in particular regular surgery to remove cancerous lesions. An estimated 1 in 450,000 individuals in Europe suffer from XP.
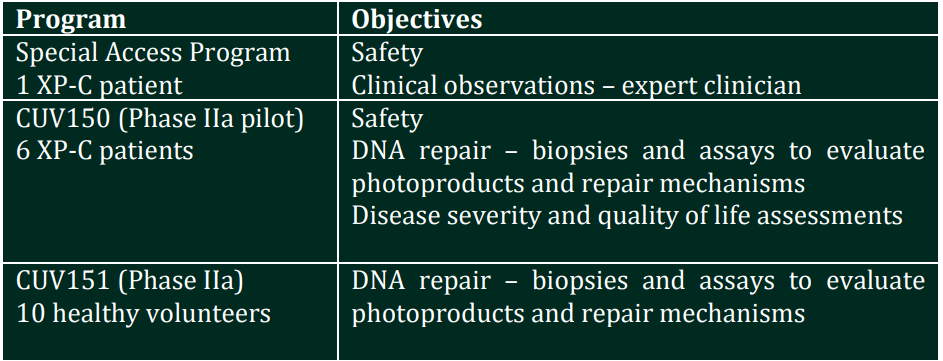
Today CLINUVEL announced that SCENESSE® has been administered for the first time to a patient diagnosed with XP under a Special Access Program, whereby the patient’s safety will be evaluated over six weeks of treatment. Following confirmation of safety of the drug product in this patient, CLINUVEL will conduct two further studies as part of the DNA Repair Program. Both studies – an open label Phase II study involving six XP-C patients (CUV150) and a control study enrolling 10 healthy volunteers (CUV151) – will evaluate the impact of treatment with SCENESSE® on DNA damage and restoration.COMMENTARY
“We seek to provide meaningful benefit to XP patients, and these results will serve a wider population of fairskinned individuals at risk of developing skin cancers,” CLINUVEL’s Clinical Operations Manager, Dr Pilar Bilbao said. “The next 12 months will be exciting for many patients, their families, the clinical experts and our own
teams.”CLINUVEL is developing a range of products based on its very targeted research with SCENESSE®, including topical formulations of its proprietary drugs and overthe-counter products, and has recently opened a new Research and Development Centre in Singapore to accelerate this work. First results from the use of SCENESSE® in XP patients are expected in 2021. The scientific data from SCENESSE® has led to further products for general use.
1 SCENESSE® (afamelanotide 16mg) is approved in the European Union as an orphan medicinal product for the prevention of
phototoxicity in adult patients with erythropoietic protoporphyria (EPP). SCENESSE® is approved in the USA to increase “painfree”
light exposure in adult EPP patients with a history of phototoxicity. Information on the product can be found on
CLINUVEL’s website at www.clinuvel.com.
Download PDF