CLINUVEL Communiqué V
| Melbourne, Australia, 09 November 2022 | ASX: | CUV |
|---|---|---|
| XETRA-DAX: | UR9 | |
| Level 1 ADR: | CLVLY |
Dear Shareholders, Friends
Introduction
(Mr Malcolm Bull, Head of Australian Operations & Investor Relations)
CLINUVEL’s key activities
Biopharmaceutical and biotechnology firms continue to be impacted by the present gloomy economic outlook. Indices and underlying share prices have, by-and-large, declined without differentiation between those that show consistent performance and the majority which are focused on pure research and development, and not near EBIT end goals. Undeterred, as part of our objective to distinguish CLINUVEL, and to insulate it from market and sectorial gyrations, we have chosen another route to earnings.
With a firm gaze ahead, the coming period, our focus remains to diversify the business while driving further development. During the various roadshows, numerous investor presentations, and face to face meetings, there have been important signs that markets start recogniszing CLINUVEL’s differentiated path, as its financial performance is exceeding that of its peers.
However, the macro reality is that recent and higher than expected inflation pummels the United States surpassing consensus forecast, and the same we see in the European Union. The current policy of monetary tightening raises the possibility of further near-term instability to global markets. In addition, while employment markets remain tense, we now have a unique spectrum of rising prices and lack of skilled workers. We expect that it will take time to reverse the trends worldwide.
The International Monetary Fund (IMF) is my ‘go to’ organisation for guidance on world economic outlook. The World Economic Outlook report of October 2022 states:
“The global economy faces steep challenges, shaped by the lingering effects of three powerful forces: the Russian invasion of Ukraine, a cost-of-living crisis caused by persistent and broadening inflation pressures and the slowdown in China”.
…in turbulent air, CLINUVEL established itself deliberately to navigate
global economic waves of uncertainty…
The IMF have not changed their forecast of world economic growth of 3.2% in 2022, but they have shaved the forecast for 2023 by 0.2% to 2.7%, with a 25% probability it could be lower. Their words are clear enough:
“More than a third of the global economy will contract this year or next, while the three largest economies – the United States, the European Union and China – will continue to stall. In short, the worst is to yet to come, and for many people 2023 will feel like a recession.”
Since the last Communiqué on 23 August, we have reported the Company’s financial results for FY2022. In this Communiqué V we:
- reflect on the results of FY2022 and the latest quareterly financial results ( 4C, September 30);
- summarise our approach to safety and quality assurance
- provide insights into our long-term approach to Healthcare Solutions products; and
- update you on key developments in communications and investor relations, including the Annual General Meeting of 26 October 2022.
Since first mentioning our global intention to present the Company to varying audiences, we have hosted, five investor meetings,across Basel, Monaco, Sydney and Melbourne (two), as well as journalist meetings and the AGM live streamed globally.
Strategic Update V was discussed and released to the ASX on 17 September 2022, following the Monaco Soirée and aimed at providing insight into the Company’s business model and odyssey. In parallel, the Company announced progress in clinical trials and provided an insightful commercial update.
An exciting addition was the unveiling of the important mission to reduce photodamage and, ultimately, skin cancer through our OTC range. Attended by Minister of Finance HE Castellini, amongst other prominent figures, the Company was well received, and appreciation of our mission was expressed by many, since the risk of skin cancer was well recognised.
I finsh up by stressing that we have had a defined plan to maneuvre the Company through a new global crisis, and coming off a high valuation in September 2021, we adopted a plan to make the Company independent of external capital. Thus far, all goes according to plan, and if we focus further we may well come out well on the other side of the economic darkness.
…we have had a defined plan to manoeuvre the company through a new global crisis…
Reflections on FY2022
(Philippe Wolgen, Managing Director)
The table below summarises CLINUVEL’s results for the financial year ending 30 June 2022.
| Variable | Outcome and Comment |
|---|---|
|
Total Revenues |
Increased by 37% to $65.7 million, including interest and other |
|
Total Expenses |
Rose 44% to $32.7 million |
|
NPBT |
Climbed with 33% to 34.3million |
|
NPAT |
Decreased 16% to $20.9 million due to a rise in taxation expenses |
Headcount |
Positive overall growth of the Group’s activities is reflected in an increase in investments and a year-on-year increase in headcount of 16% |
Net Assets |
Increased by 27.2% to $125.6 million with the key balance sheet change being a substantial rise in cash balances of $38.8 million to $121.5 million. (Cash at 30 September 2022 increased to $137.6 million.) |
Other Key Performance Measures |
Return on Equity: 17%. Earnings Per Share: 42.3 Australian cents |
Dividend |
Declared a fifth consecutive annual dividend which is a 60% increase from 2.5 cents last year to 4.0 cents on FY2022 earnings |
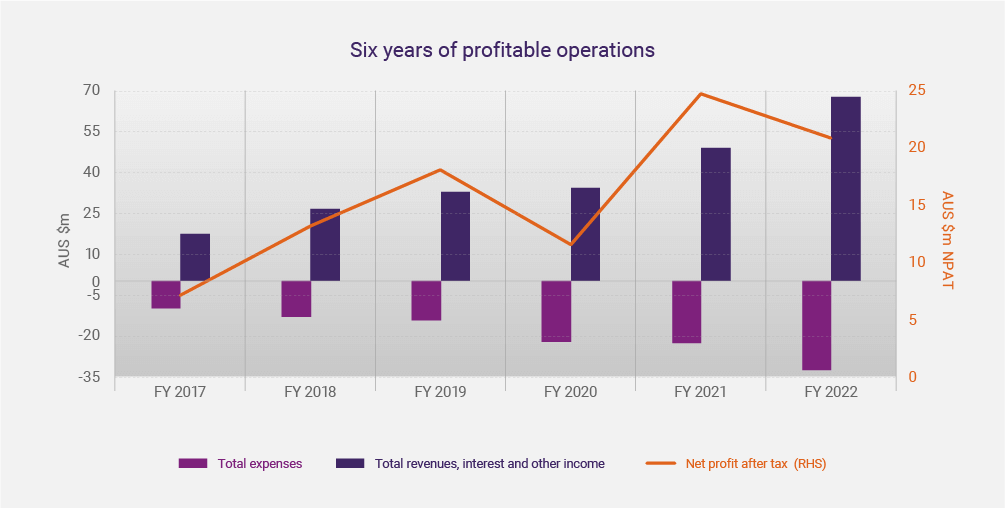
A sixth consecutive year of profitability provides just one reason to halt for a moment and reflect. We are at a fascinating but critical juncture in our history. Arguably, one could continue the strict focus on current commercial programs, and or use our current operational base and cash reserves to expand the Group. It is also relevant to review the last six years’ results, as presented in the Investor Webinar on 30 August 2022
Pause to Reflect
For long, we have made known our wishes to lay a foundation and infrastructure which would enable us to integrate new teams, new technologies to expand our foothold. At this very moment in time, I believe that the Group is now robust enough to go through further expansion, absorbing new teams cross-border and operate on a larger footing.
The basis for this belief stems from the calibre, growth, and depth of our executive management team as well the senior management team. Having recently attended off-site meetings and workshops with the executive management, I witnessed its profound knowledge, details of operational experience, commercial nous and strategic execution in the United States and Europe. Our Company relies on the centrefold team which has worked together for more than 15 years. The understanding and flawless interaction between these executive managers is really a result of both longevity and a joint will to win within a harmonious working environment. This team forms the engine of the Company and, having remained focussed on common goals, the operating procedures and automatisms offer a unique opportunity to expand this modus elsewhere, new domains, new markets, and varying technologies.
…our Company relies on the centrefold team which has worked together for more than 15 years
In the meantime, at the core of our development remains the family of melanocortins – specific physiologic hormones to be used for unaddressed populations – aiming for a vast addressable market in the North America and Europe.
I spoke earlier that our teams are now all on a countdown clock, more than ever aware that speed and accuracy of execution matters, with the bulk of work and value drivers to be realised ultimately by June 2025.
September 30 (Q1 FY23) Quarter: Growth
Changing views, the Q1 FY23 (unaudited) financial results released at the end of October provide a fair indication as to the business progress since 1 July. Despite no longer needing to conform to the Appendix 4C requirement, we recognise that many investors – particularly those based outside Australia – expect companies to offer financial visibility on a quarterly basis, and therefore we align our reporting cycles to accommodate those.
As demand for SCENESSE® increases, we continue to see growth in both Europe and North America. In a commercial update in Q1 2023, we will expand on our activities preparing future markets.
In parallel, in line with our expressed plan to invest $175m in the growth of the business for the five-year period to 30 June 2025, we see a controlled increase in expenses. At the same time, our growing cash reserves – $137.65 million as of 30 September 2022 – form the armamentarium to withstand systemic shocks, not distracting us from our objective of growing the group.
…the armamentarium to withstand systemic shocks…
Results forthcoming
The first three patients in CUV156 have completed the trial, and with much anticipation we await the interim analyses performed and to be released by the specialised laboratories. As reported, XPC and XPV patients are intolerant to UV and visible light sources as they critically miss the capacity of nucleotide excision repair, the inability to repair DNA skin damage caused by the slightest exposure to UV.
The XP program holds much relevance, as we aim to confirm in human studies that melanocortin’s positively affect and assist DNA repair mechanisms in photodamaged skin. There is great relevance of being able to use data generated from prescriptive drugs and translating these to a non-prescription range of products.
The Division of Healthcare Solutions is advancing its work on these products – what we call OTCs – benefiting the Highest Risk populations, those most prone to photodamage and skin cancers. The first product CYACÊLLE® is being evaluated by a select number of users, communities of the immune compromised and suppressed patients. The overall aim is to obtain feedback on the properties of this first formulation manufactured.
In parallel, the CBM teams are evaluating the first digital campaign to understand the efficiency and depth of content among our communities of interest. The second campaign is being prepared, while new ambassadors (CUVAs) are being contracted. Overall, the CUVA program is designed to disseminate CLINUVEL’s mission among the ‘Highest Risk’ populations by raising awareness. More and frequent news will come our way the next 12 months.
In parallel, the ACTH manufacturing is progressing, and based on the data and information obtained from the chemical facilities, our teams are confident we will be submitting our first dossier to regulatory authorities in 2023.
The further clinical trials in stroke and vitiligo will be discussed in January 2023.
Passing of Dr Kendall Marcus, Director Division Dental and Dermatology Products, FDA
With intense sadness, we learned of the sudden passing of Dr Kendall Marcus. She was known to us as a genuine and devoted physician to better the lives of patients in the USA. An intelligent woman, and without doubt the most studious Director this Division has had, she was driven and dedicated to bringing innovative medicines to the market.
The first encounter with Kendall was in 2015, as she initiated a telephone call to me to discuss SCENESSE®, after she had learned that the drug had been approved by the EMA. She came across as inquisitive and genuine, and the hour-long conversation led to our commitment to submit European data to her Division prior to a formal FDA submission.
During the various interactions with Kendall in later years, it was apparent that she was truly interested in novel medicinal therapies, simply put she would look where others would not have. Over the years, our teams had had our moments of professional disagreement – specifically on vitiligo, and the need to treat patients of darker skin colour – but these exchanges were always cordial and on those occasions left by Kendall with the promise to further read and study about the proposed route.
Presided by Dr Marcus, in October 2019, SCENESSE® obtained FDA approval. She took pride in shepherding through afamelanotide as the first new chemical entity approved by her Division.
I am convinced that Kendall would have liked to see systemic innovation coming to the US for vitiligo patients, as she initiated the first FDA-hosted Townhall Meeting for vitiligo patients in March 2021. Unfortunately, time was not granted to her to see the beneficial outcome for patients. I wish to see our teams remaining inspired by Kendall’s vision and determination.
My thoughts go out to her husband and family she has left behind.
Delivering Healthcare Solutions
(Emma Dyer, Head of Brand)
Healthcare Solutions for New Audiences
As a global authority in photomedicine, CLINUVEL has embarked on a mission to raise awareness to reduce photodamage and global skin cancer among populations most prone, and at highest risk.
Last year, I joined a company with a LASER focus on researching melanocortins for multiple indications. With a suite of new active substances, part of the same family of hormones, a new chapter had started to make these available in specialised products, part of the range of non-prescription products. Therefore, I was faced with a pharmaceutical company expanding its repertoire in targeted consumer care, led by a new franchise within the Company.
The next phase is preparing the launch of new products with a combination of active and natural substances for those in high need of skin protection.
My professional adventure at this company is one marked by trying to solve problems for many of us with unanswered needs, namely immunosuppressed, skin cancer susceptible, and those who endure extreme outdoors. I know all too well that the objective of any commercial venture is to differentiate it from any other competitor, or in CLINUVEL’s case those that are in the same market yet are not so close to our mission of solving health problems. I started from day one to set up a team to deliver on several initiatives.
Our spectrum of products consists of three product lines – polychromatic photoprotection, assisted DNA-repair and pigmentation stabilisation. As said, the first product of the polychromatic photoprotection line is currently in testing phase and is expected to be launched in 1H 2023. The polychromatic photoprotective screen translates our expertise in developing treatments for EPP and photo-skin-diseases severely affecting patients from the harmful effects of photons, light consisting of the invisible and visible parts – UV light, blue, green, and beyond.
This third generation of Polychromatic Screens (PCS) protects against all wavelengths of solar exposure and, as the name suggests, a wide spectrum of light UVB-UVA-HEV (blue and green spectrum of light). Complying with regulatory standards, all novel products need to list an equivalent of SPF 50+ (a regulatory connotation).
The first product CYACÊLLE ® is now part of the third-generation research in polychromatic light impairment (λ3), containing mineral filters and other excipients to scatter light and, more importantly, plant phyto-actives reducing the penetration of High Energy Visible Light or Blue Light by 70%. Over 65% of ingredients are natural as we seek to preserve marine life and biology, whilst leaving skin replenished.
…our spectrum of products consists of three product lines – polychromatic photoprotection, assisted DNA-repair and pigmentation stabilisation…
This specific combination is clinically proven to protect skin from penetrating photons along 308 nanometres up to 500, aiming to reduce photodamage. Following the first-generation product, we will scale up production in a second-generation product, which boasts reflective and refractive properties facilitating additional photoprotection in multiple shades; our intention is to make these available mid-2023.
Down the line, as the results from our pharmaceutical program in xeroderma pigmentosum are coming through, we will reveal our first assisted DNA-repair product line. It has remained central to our R&D efforts that the data from our pharmaceutical programs provide justification for the non-prescriptive products. As an example, the XP clinical work and findings flow over in our research, being translated into an assisted DNA-repair serum which supports our natural repair function, and unlike other products contain the active substances which are clinically proven to do just that.
With a mandate to bring full circle a journey that had started many decades ago in using melanocortins, our CBM teams are putting efforts in to build wider audiences. As we prepare the launch of three targeted product lines, it is easy to accept that products without audiences are not going to be successful. Vice versa, we see many products coming to market with less or no innovation, but having audiences already. Eventually, the dermato-cosmetic products will complement our pharmaceutical ones in vitiligo. I have had much time to study history and a plan for us to come full circle, and held in fascination we are building that every day.
…first product CYACÊLLE® is now part of the third-generation research in polychromatic light impairment (λ3)…
Direct to Consumer (D2C) Distribution
Aligned with the Company’s overall commercial strategy of vertical integration, the OTC product range will be launched using a D2C model favouring a ‘pull’.
We looked at the Lifetime Value of capturing an interested audience, that was our starting point. In addition, I acknowledge early on that the red line running through the Group is very much a longitudinal approach to providing care and services. In my field, I am used to incorporate corporate values as part of the overall brand and message one wants to communicate, therefore we will see these coming through in our campaigns and choices of CUVAs.
As highlighted, both our approach to care and products are differentiated: we started from the medical field as a global authority in photomedicine. For the healthcare consumer market, we chose a ‘pull’ marketing strategy at the outset.
Our approaches consider a broad range of facets of targeted digital marketing, with a view to creating and delivering an outstanding online customer journey. It includes search, website, and compelling organic content across all social media, resulting in a strong online presence and clear positioning.
Our ‘demand-driven’ marketing strategy, unsurprisingly, remains true to our focus on addressing those who have been overlooked. As mentioned above, our large, specialised audiences with unmet needs are first comprised of “Immunocompromised, Skin Cancer Susceptible and Extreme Outdoors”. These groups require and, we have learnt, demand deep information on solar protection, as well as the next generation of solar care; for understandable reasons, to date they have remained unaddressed by the cosmetic conglomerates.
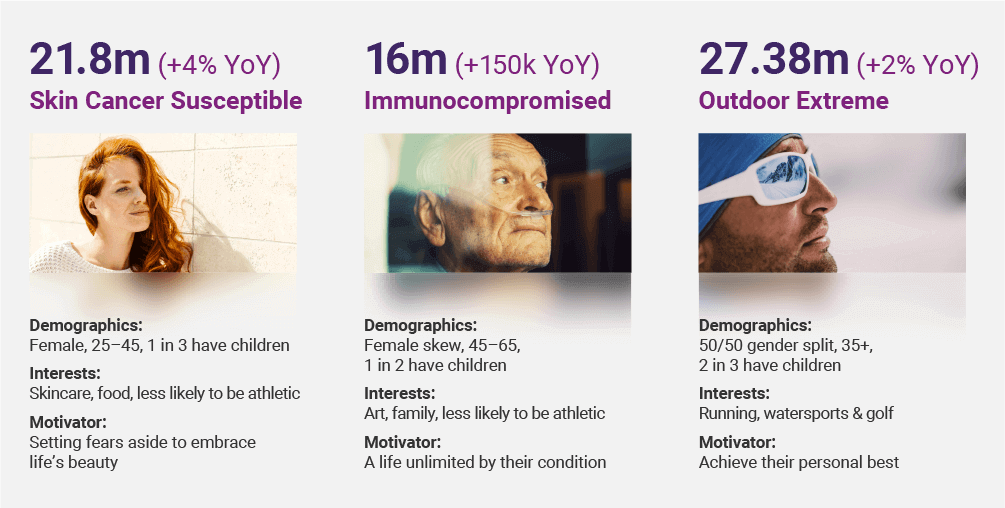
CUVAs and CUVIPs
Unifying these globally fragmented audiences is the challenge we have taken on. Our somewhat disruptive CUVA (CLINUVEL Ambassador) Programme has been designed to do just that. Built for the long-term, our model steps away from the traditional influencer approach, favouring authenticity and longevity. Selecting thought leaders from our Highest Risk groups, we are giving them a voice, increasing awareness, culminating in a global community of 35 million.
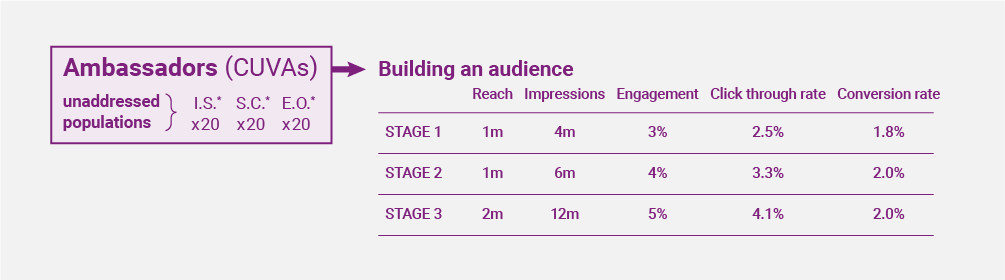
During our first online campaign, the first nine CUVAs delivered the ‘Untold Story of Light’ campaign, reaching over half a million of our target audience in just ten weeks. This programme will scale over the coming months as we aim to engage 30 CUVAs by the end of H1 2023, and 60 by the end of the year. In 2024, we will retain all 60 CUVAs with equal representation across our Highest Risk groups. The CUVAs will tell authentic stories, educate their audiences on risk, whilst advising them to learn more from CLINUVEL’s solar mission. The result of this will be over 35 million impressions with an estimated average CTR of 4.5% and conversion rate of 2%, no mean feat and a significant commercial opportunity.

Such is the scale of our ambition, now we have extended the CUVA programme to include CUVIPs. These are unique and intriguing personalities who are prominent and respected in their fields. It is a two-tiered programme with CUVIPs who have more than one million followers (tier 1) and those with less than one million (tier 2). CUVIPs sign up for a minimum of one year and up to two years dependent on seniority and following. They enter our business as shareholders, becoming CLINUVELLIANS. CUVIPs will promote our shared mission, following a structured communication plan which includes, events, interviews, and e-mails. This programme will deliver an incremental reach of ten million.
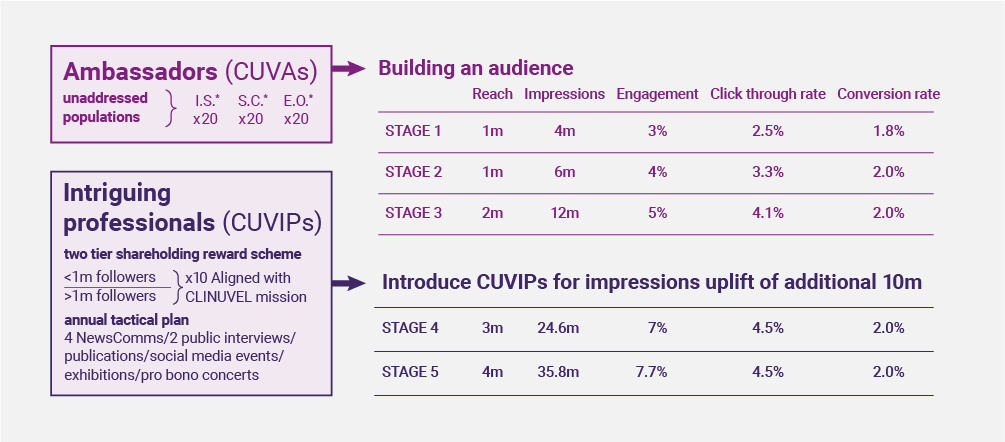
This approach steps perhaps slightly away from the usual found online from representation of affected people, it is certainly differentiated way of seeing our future, but it works thus far. Doing good comes first, the rest follows.
Quality Assurance and Safety
(Azza Hamila, Head of Quality and Drug Safety)
Continuous Improvement: a Pillar of Total Quality Management
As head of Quality, I share with you the background of my discipline.
After the Second World War, Japan was almost ruined. The country was in a vicious cycle of poor industrial development with Japan’s poor product quality being the principal obstacle to sustainable international trade. It was time to explore new ways of thinking about quality and this change in mindset was crucial towards Japan’s economic turnaround. Through research, teaching, and the application of best practices in quality management, Japan developed the new “total quality” approach: rather than relying purely on product inspection, Japanese manufacturers focused on improving all organisational processes through the people who used them. As a result, Japanese manufacturers were able to produce higher-quality products at lower prices, and quickly positioned themselves as world leaders in quality.
One of the pillars of the total quality management concept is continuous improvement: the company’s effort to constantly increase the ability to fulfil requirements to highest industry standards. This is achieved through the use of quality policies, quality objectives, analysis of data, corrective and preventive actions, management review, and audit results. It is a structured approach which should become part of the mindset and culture of an organization, to which a firm and its staff can aspire.
The Importance of Audits
In the pharmaceutical industry, audits are regarded as means of evaluating compliance with the specified objectives as defined in the company quality system. As such, quality audits in the pharma sector provide a pathway to ensuring continuous improvement by offering relevant feedback to the company and management. There are generally two types of audits: internal and external.
Internal audits are repeatedly carried out by a company on its own system, procedures, and facilities. The objective is to evaluate the existing activities and documentation, and determine if these meet the established standards.
An internal appraisal process will evaluate the strengths and weaknesses of the processes, the results of which will help build a better system for the benefit of the company. Internal audits in Quality and Pharmacovigilance are used as a means of keeping the processes under control and be “inspection” ready at all times.
External audits refer to a customer conducting an audit on a supplier or on outsourced activities. The contractee, the customer – supplier of the mandate – is responsible for assessing the overall competence of the contract acceptor according to relevant guidelines but also against the contractual agreements (e.g., Quality Technical Agreements, Safety Data Exchange Agreements). External audits, also called third-party audits, also have the advantage to develop knowledge and confidence in the partnership agreement. The key to the success of both types of audits is preparation, preparation, and preparation to withstand qualified and independent auditors, and close monitoring of the audit results and their remediation.
Regularity in auditing is essential in maintaining compliance status. Audit schedules at CLINUVEL are determined through a risk-based management process where each process, operation, or supplier is assigned a risk “rank” based on a range of factors, such as their previous audits and criticality to business continuity. As an example, high-risk operations usually include sterile manufacturing, primary packaging, and labelling and final product release testing, which are subject to more regular audit. These operations tend to have a higher degree of regulatory oversight and are therefore assigned a higher risk level. Additionally, supply chain oversight including distribution and transport practices are regarded as a high risk in today’s environment. Medium-risk operations would include solid oral dosage form manufacturing, while warehousing (e.g., storage areas for product and raw materials) could be considered low-risk operations if appropriately controlled. But some risks are relative as they will depend on the company’s business profile. The risk profiles will then vary depending on several factors to consider like site information (e.g., facility design, equipment maintenance), the product class (e.g., hormones), the formulation type (e.g., injectable vs. oral solid dose) or the compliance status since last audit.
The Role of Audit Management Software
Auditing is an excellent indicator of the overall effectiveness of the Company’s and the contractor’s quality systems and product performance. Implementing risk-based approaches to the Company’s audit program helps to focus resources on key areas of importance, thereby minimising the risk to patients. Using Audit Management Software solutions or eQMS can further improve the Company’s audit programmes by automating all audit-related activities, collecting documents and reports across the life cycle in a single location or linking the responses to the findings making is easy to track and resolve quality issues.
Summary
The quality of a pharmaceutical product must be maintained through multiple stages from manufacturing, storage and handling and administration to the patient. Risks in all areas of the process need to be managed proactively and effectively to ensure smooth operations and the seamless provision of the drug to those in need. Continuous improvement is becoming part of the mindset across the business, with systems in place and subject to constant review to ensure compliance, continuity of business and, above all, patient safety.
As of today, under my guidance, the Company has come through more than 10 external audits, and it is owing to all our staff that we understand the significance of the processes to be maintained within the Group. There is much value embedded in these.
Peer Review Publication and Conferences
(Lachlan Hay, Director of Global Operations)
Following the launch of SCENESSE® for EPP in the USA in 2020, patients have been treated and followed up by Specialty Centers, with over 40 Centers now active across the country. Through this network, a standard of care has been established for EPP while Centers collect data on the safety and effectiveness of treatment as part of long-term patient follow-up.
The first data from the use of SCENESSE® post-marketing in the USA was published earlier this month by the teams at Henry Ford Health System (HFHS) in Detroit. Across a cohort of 22 adult EPP patients, the HFHS team reported significant improvement in patient quality of life – as measured by the disease-specific EPP-QoL tool – across all patients, compared to baseline, with the greatest improvement noted after the first implant and incremental improvement with consecutive treatment. Variability in patient response led to the authors suggesting attention be paid to more individualised treatment strategies for patients. The paper adds to the growing body of real-world evidence published from the post-marketing use of SCENESSE® , with previous data reported from patient cohorts across Europe.
Data from the CUV801 study – investigating the safety and efficacy of afamelanotide in six adult patients who suffered arterial ischaemic stroke – was recently presented to the 14 th World Stroke Congress in Singapore. Two separate poster presentations focused on the study design and results; the first time the program has been presented to a global academic audience. One poster focused on the results of magnetic resonance imaging, measuring the extent of fluid retention in the brain following the stroke and its treatment and reporting an overall reduction in damage over nine days under medical supervision. Full results from the CUV801 study are currently being submitted for peer-review publication.
The publications referred to are:
- Ceresnie, M.S., et al., (2022). Association of quality of life measures with afamelanotide treatment in patients with erythropoietic protoporphyria and x-linked protoporphyria: A retrospective cohort study. Journal of the American Academy of Dermatology, S0190962222028729.
- Stanislaus V., et al., (2022). Signal of efficacy of afamelanotide in acute stroke patients – an open label, proof of concept, Phase IIa study. World Stroke Congress 2022. Singapore. 26-29 October.
- Stanislaus V., et al., (2022). Feasibility and safety of afamelanotide in acute stroke patients – an open label, proof of concept, Phase IIa study. World Stroke Congress 2022. Singapore. 26-29 October.
Communications and Investor Relations
(Malcolm Bull, Head of Australian Operations and Investor Relations)
Recent Company announcements
The Company’s recent announcements are listed below:
| Date | Announcement |
|---|---|
|
30 Aug |
Appendix 4E and Annual Report 2022 |
|
31 Aug |
CLINUVEL Investor Webinar Financial Results 2022 |
|
05 Sep |
CLINUVEL Submits Label Expansion for Adolescent EPP Patients |
|
08 Sep |
Annual General Meeting Date |
|
13 Sep |
Presentation – H.C. Wainwright Global Investment Conference |
|
19 Sep |
Strategic Update V |
|
23 Sep |
Notice of Annual General Meeting / Proxy Form |
|
13 Oct |
Corporate Presentation – Sydney Soirée |
|
19 Oct |
First Vitiligo Patient Enrolled in Monotherapy Study |
|
21 Oct |
Melbourne Investor Briefing Presentation |
|
26 Oct |
Chair’s Address and Results of Annual General Meeting |
|
31 Oct |
Appendix 4C & Activity Report |
|
09 Nov |
News Communiqué V |
All CLINUVEL’s announcements are available on the CLINUVEL website and CLINUVELNews. More specifically, announcements to the Australian Securities Exchange are available on the investor pages of the CLINUVEL website.
Shareholder Feedback on FY2022 and Events
The financial results for the year ending 30 June 2022 were very well received. The sixth consecutive year of revenue growth and profitability and the fifth consecutive year of dividends contrasts with the bulk of the listed life-science companies in Australia but also elsewhere that are research and development milestone based.
Shareholders have welcomed the Soirée series of gatherings for the information imparted on CLINUVEL®. As stated above, we have now held Soirées in Basel in May, Monaco in September, and Sydney in October. The Sydney event on 13 October had a mix of attendees, spanning many analysts, various bankers, and brokers, as well as long-standing shareholders and members of the broader financial community. They received briefings from Malcolm Bull, Lachlan Hay and Philippe Wolgen and a thorough question and answer session concluded the evening. We announced a summary of the presentations to the ASX on 14 October for the benefit of all stakeholders. As we hold Soirées in other places around the world in 2023, we will continue this practice.
Annual General Meeting 2022
The Company hosted its 2022 Annual General Meeting on 26 October, the first “in-person” Annual General Meeting since 2019 and the first time we have live streamed the event online.
To provide shareholders with a broader understanding of the business, the Company chose to host a panel Q&A session in lieu of the traditional Managing Director’s address to the AGM. We are grateful to Dr David Stanton of Jefferies for hosting this interaction, which sought to address questions received on notice prior to the meeting and covered a wide range of compliance, regulatory, clinical, and operational issues across CLINUVEL. Feedback on this format has been positive and we are considering how we can build on this for future events.
For the second consecutive year, the Company received over 22 million votes in resolutions, showing a high level of engagement with the shareholder base, while all resolutions passed in line with the recommendations of the Directors.
For shareholders who were unable to attend, a copy of the livestream is now available on the CLINUVELNews website.
Summary and conclusion
Challenge and uncertainty go hand-in-hand, and this has never been more heightened than in the current economic environment. Due to its deliberate and focused long-term strategy and business model, CLINUVEL® is in a stronger financial position than before and has expanded its team to manage unforeseen challenges ahead. We maintain the pace of distribution of SCENESSE® to secure ongoing net cash inflows to self-finance the expansion of the clinical program, the drug portfolio, and the path to commercial launch of the first Healthcare Solutions products. These are the catalysts that will spur the value of CLINUVEL® in the market but should expect volatility until monetary authorities and governments control inflation. We welcome the support of shareholders on the journey as we are moving further ahead towards a diversified and sustainable pharmaceutical group.
We wish you all health and wisdom.
Thank you for your support.
Malcolm Bull, Head of Australian Operations & Investor Relations
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD.
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products; the COVID-19 pandemic and/or other world, regional or national events affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical products; competition for our products, especially SCENESSE® (afamelanotide 16mg), PRÉNUMBRA® or NEURACTHEL®; our ability to achieve expected safety and efficacy results in a timely manner through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, Israel, China and Japan of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE®, PRÉNUMBRA® or NEURACTHEL® which may lead to it being unable to supply its commercial markets and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology and consumer based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; our ability to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2021 Annual Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on preliminary and uncertain forecasts and estimates is available on request, whereby it is stated that past performance is not an indicator of future performance.
Contact
Level 11, 535 Bourke St
Melbourne, 3000 Vic,
Australia
+61 3 9660 4900
+61 3 9660 4909