Chairman’s Letter II
31 MAY 2021
Update during 2021
In a year with many ups and downs, the COVID variants has slowed down world businesses and also CLINUVEL’s clinical development in hospitals. However, the supply of SCENESSE® has shown a straight way up as we showed in the last quarterly analyses. In my best Dutch translation, I will present you my view on CLINUVEL’s 2021 operations.
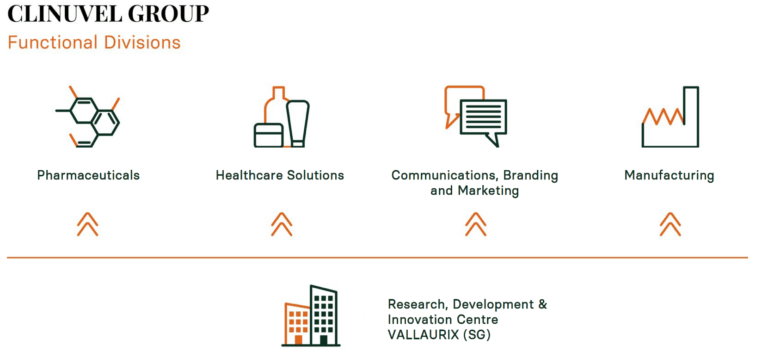
This Board agreed in 2019 that CLINUVEL would need to have the blueprint of a diversified group, and we are on our way to realize this goal. All directors are enthusiastic by all the activities performed at the same time, very different from 5 years ago when our team was focussed on one drug in one disease. Now, there is research in 5 diseases, 2 pharmaceutical products, healthcare product lines to add to our medical products and a whole new communications-branding-marketing team. New markets, new products and new communication will lead to growth, I have seen this in many of my businesses. I am happy about our senior managers’ initiatives, and many discussions are held on the future of DNA repair. During the last period of COVID, the managers have worked from all places in the world to grow the development pipeline of CLINUVEL’s products, formulations and new healthcare solutions.
When speaking to my trading partners asking about my role in CLINUVEL, I have to simplify the story. I try to tell our new German and Dutch investors that we specialize in hormones that repair and protect the skin and brain. As our executives say, the skill is to communicate all these activities coming from 4 divisions so that the great public get to know CLINUVEL.
CLINUVEL has attracted the attention of pharma press looking at how the Company will enter a wider non-medical market since it is originally coming from a pharmaceutical specialty. Also, many questions have been asked on the new indication for SCENESSE® and total value of all the projects. I can only say that our teams concentrate on one job without distraction, and this will produce long term results. They have done this for more than a decade, and they continue to perform, only time is needed. This group of managers and directors are sure that all together, products and services and people will lead to a scaled-up company.
One of my main tasks as Chairman is to ensure the continuation and the completion of research by keeping leadership and knowhow in CLINUVEL in a positive environment. We are working together with lawyers and remuneration advisors to see what we can do to keep key managers in the Company.
I believe that the same team who built the Company from A$40 million to today’s stock value should continue, because the cost of the change will be too expensive and providing many uncertainties. I am responsible for the long-term strategy of the Company and therefore the directors need to take decisions to build value for all, not only a few shareholders who vote. The discussions on continuation of management are ongoing with our CFO and CEO about the future.
This week I will be discussing CLINUVEL’s future and operations in interviews with Dutch business analyst Professor Kitty Koelemeijer and political commentator Mr Jort Kelder. I understand from our teams, that these interviews have English subtitles and will be available in a few weeks.
My final goal is to see CLINUVEL lead as a melanocortin house, a team who will have various hormones from this family on the market and products for healthcare users.
I also look at other global business in retail, travel and trade and these are all suffering and losing value, therefore we can look back at 17 months of satisfaction when assessing CLINUVEL’s operations and how our managers find solutions. Thus, our directors are optimistic that more problems will be solved in the future. I will speak to you in September and until that time there will be many research and commercial activities of our promising and growing company.
Willem Blijdorp
Chair
CLINUVEL PHARMACEUTICALS LTD
– End –
Authorised for ASX release by the Chair of CLINUVEL PHARMACEUTICALS LTD
About CLINUVEL PHARMACEUTICALS LIMITED
CLINUVEL PHARMACEUTICALS LTD (ASX: CUV; NASDAQ INTERNATIONAL DESIGNATION ADR: CLVLY; XETRA-DAX: UR9) is a global and diversified biopharmaceutical company focused on developing and commercialising treatments for patients with genetic, metabolic, and life-threatening disorders, as well as healthcare solutions for the general population. As pioneers in photomedicine and understanding the interaction of light and human biology, CLINUVEL’s research and development has led to innovative treatments for patient populations with a clinical need for systemic photoprotection, DNA repair and acute or life-threatening conditions. These patient groups range in size from 5,000 to 45 million worldwide. CLINUVEL’s lead compound, SCENESSE® (afamelanotide 16mg), was approved by the European Commission in 2014, the US Food and Drug Administration in 2019 and the Australian Therapeutic Goods Administration in 2020 for the prevention of phototoxicity (anaphylactoid reactions and burns) in adult patients with erythropoietic protoporphyria (EPP). More information on EPP can be found at http://www.epp.care. Headquartered in Melbourne, Australia, CLINUVEL has operations in Europe, Singapore and the USA. For more information please go to http://www.clinuvel.com.
SCENESSE® and PRÉNUMBRA® are registered trademarks of CLINUVEL PHARMACEUTICALS LTD.
Head of Investor Relations
Mr Malcolm Bull, CLINUVEL PHARMACEUTICALS LTD
Investor Enquiries
https://www.clinuvel.com/investors/contact-us
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance, or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products, the COVID-19 pandemic affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical products; competition for our products, especially SCENESSE® (afamelanotide 16mg); our ability to achieve expected safety and efficacy results through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, China and Japan of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE® which may lead to it being unable to supply its commercial markets and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; any failure to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2020 Annual Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on the forecasts and estimates is available on request. Past performance is not an indicator of future performance.
Level 11
535 Bourke Street
Melbourne – Victoria, Australia, 3000