Afamelanotide well tolerated by first stroke patients
| Melbourne, Australia, 10 August 2021 | ASX: XETRA-DAX: NASDAQ INTERNATIONAL DESIGNATION: |
CUV UR9 CLVLY |
Editorial note: CLINUVEL has today released both a Scientific Communique (www.clinuvel.com) and a short video (news.clinuvel.com), explaining the pathology and treatment of stroke.
Afamelanotide in Arterial Ischemic Stroke – Phase II Study CUV801
Executive Summary
- three stroke patients treated with afamelanotide, no treatment related adverse events reported
- all three patients had a cardiovascular history prior to the stroke
- afamelanotide treatment of clots in the higher regions of the brain
- no significant side effects due to afamelanotide administration
- two patients significantly improved (NIHSS), one no improvement
CLINUVEL’s drug afamelanotide was well tolerated by the first three arterial ischaemic stroke (AIS) patients enrolled in a world first clinical trial (CUV801). The patients experienced acute strokes due to a clot formed in the higher regions of the brain and were treated with afamelanotide at a specialist neurological hospital in Australia. None of the patients experienced drug-related adverse events. All patients have been discharged from critical care.
In total, six adult AIS patients are to be evaluated in the CUV801 study. The study focuses on the safety and therapeutic potential of afamelanotide in patients who are ineligible for standard stroke therapy. AIS accounts for approximately 85% of the 15 million strokes suffered worldwide each year, while more than 85% of these patients are ineligible for standard of care treatment.
“The first meaningful learning of this study is that stroke patients with a cardiovascular condition are able to receive afamelanotide without significant adverse reactions,” CLINUVEL’s Head of Clinical Operations, Dr Pilar Bilbao said. “On the basis of the first three patients, there is a prospect that CLINUVEL will be able to treat many more stroke patients than initially anticipated, since one third of stroke patients globally have underlying heart problems.
“In being the first to use a melanocortin in stroke patients, our team and physicians observe the tolerance to frequent dosing of afamelanotide in patients who suffer a life-threatening condition. We are delighted to report that one patient was rapidly discharged from specialist care, while the other two patients are still early in their recovery and rehabilitation programs,” Dr Bilbao concluded.
Afamelanotide in First Stroke Patients
In following CUV801 study protocol, all three patients underwent close clinical monitoring during hospital stay. During this period, the neurology specialist team responsible for patient care assessed the safety of intervention with multiple doses of afamelanotide as well as changes in neurological function and the extent of disability.
Afamelanotide treatment was well tolerated by the patients, with no reports of drug-related adverse events. All patients received intermittent treatment with afamelanotide per the CUV801 protocol.
Patients’ neurological functions were assessed clinically using the Modified Rankin Scale and National Institutes of Health Stroke Scale (NIHSS), while imaging of brain tissue is used to evaluate the extent of changes within the brain (CT perfusion¹ and MRI²).
Two patients showed improvement in neurological deficit (NIHSS), while one patient showed no improvement.
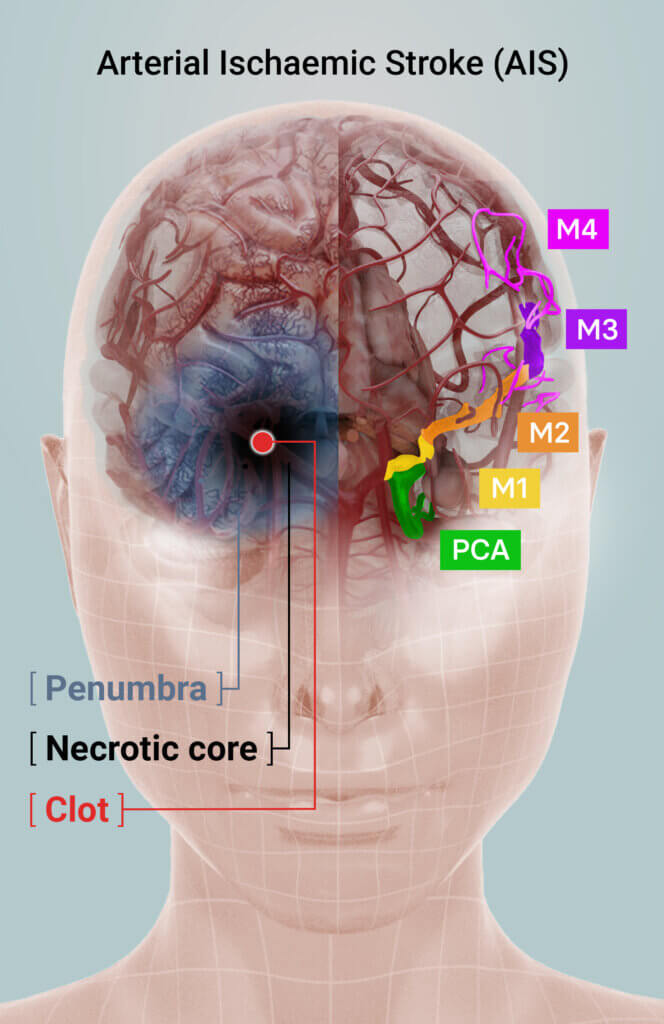
During the assessment of MRI imaging, special focus is on the blood flow (perfusion) to the affected regions of the brain (core and penumbra) following afamelanotide treatment.
Arterial Ischaemic Stroke (AIS) Treatment
An ischaemic stroke is caused by a clot lodged in a blood vessel in the brain, which restricts blood flow, oxygen and supply of other nutrients to surrounding tissue. Tissue in the zone around the clot – the necrotic core – is irreparably damaged. The area surrounding the core – the penumbra – is at immediate risk of further tissue death but can be returned to normal function if immediate intervention can be offered.
Stroke treatment aims to restore blood flow to the brain quickly to salvage tissue in the penumbra. Current therapies either chemically dissolve and/or physically remove the clot but must be administered within strict therapeutic windows or risk exacerbating the severity of the stroke or subsequent disability.
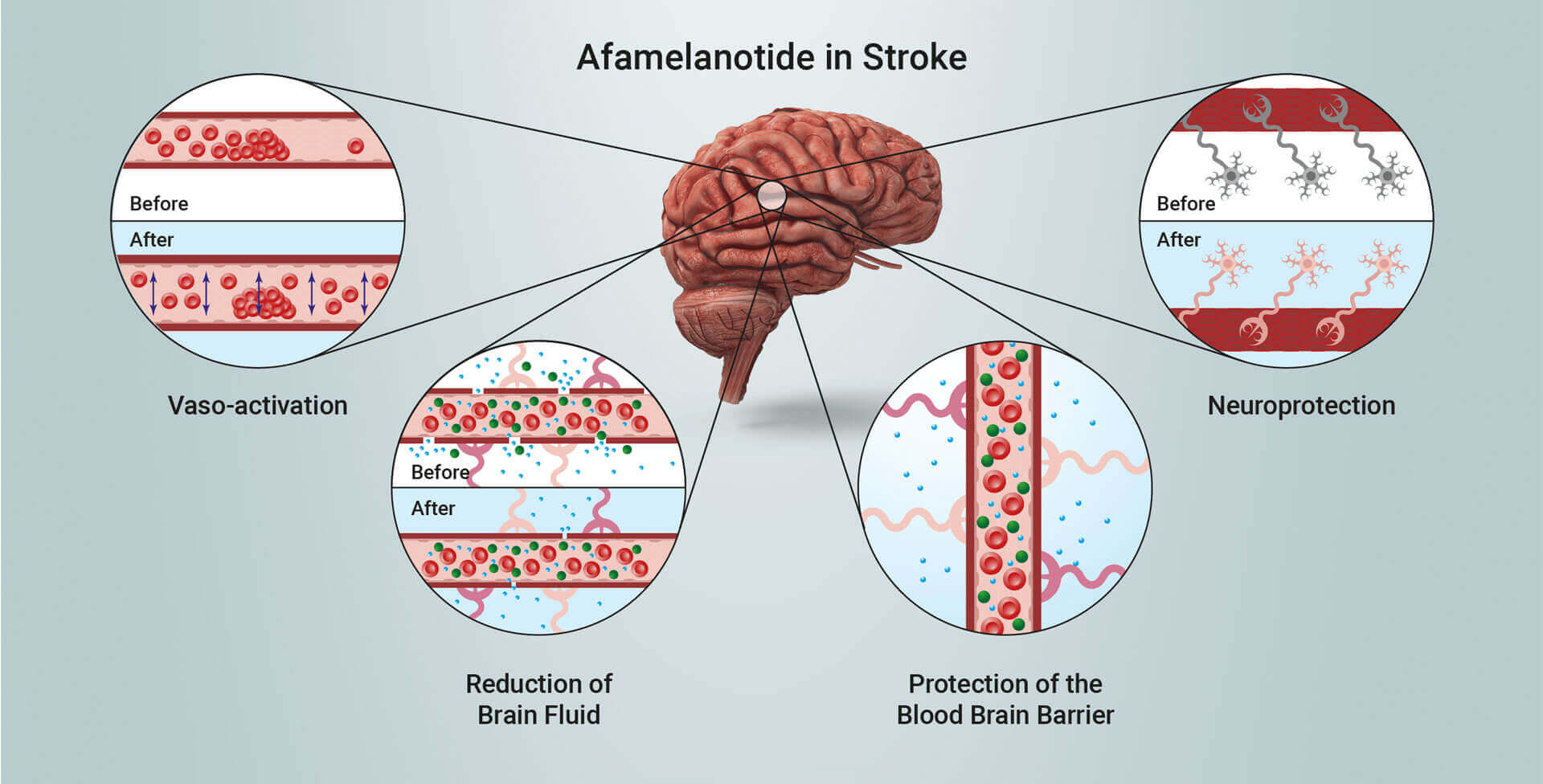
Scientific progress has demonstrated melanocortins, including afamelanotide, provide a positive effect on the central nervous system (CNS). Afamelanotide is known to offer neuroprotection and act as a potent anti-oxidative hormone. The drug possesses further potential therapeutic benefits, activating blood vessels, reducing fluid formation, protecting critical nerve and brain tissue, and alleviating the disruption of the blood brain barrier (BBB: a critical defence mechanism protecting the brain). Afamelanotide is expected to positively affect the blood flow and oxygen supply to deprived brain tissue following stroke.
1 Computerised tomography
2 Magnetic resonance imaging
– END –
About Acute Stroke
Stroke is the second most common cause of death and a leading cause of disability worldwide, yet many stroke patients are unsuitable to receive the current standard of care (clot removal and clot dissolution). AIS accounts for approximately 85% of the 15 million strokes suffered worldwide each year. Despite its prevalence, treatment options are limited: in Europe, over 85% of AIS cases presenting to hospitals are not eligible for current standard of care treatment (thrombectomy and thrombolysis).
About Afamelanotide
Afamelanotide exhibits neuroprotective, vasoactive (acting on blood vessels), anti-oncotic (anti-swelling) and anti-oxidative effects by optimising blood flow and reducing the size of injury and fluid (oedema) formation. The drug has been shown in non-clinical models to restore the flow of blood and oxygen to the brain after stroke and reduce the extent of cerebral damage. SCENESSE® (afamelanotide 16mg), was approved by the European Commission in 2014, the US Food and Drug Administration in 2019 and the Australian Therapeutic Goods Administration in 2020 for the prevention of phototoxicity (anaphylactoid reactions and burns) in adult patients with erythropoietic protoporphyria (EPP). More information on EPP can be found at https://www.epp.care.
About Clinuvel Pharmaceuticals Limited CLINUVEL
PHARMACEUTICALS LTD (ASX: CUV; NASDAQ INTERNATIONAL DESIGNATION ADR: CLVLY; XETRA-DAX: UR9) is a global and diversified biopharmaceutical company focused on developing and commercialising treatments for patients with genetic, metabolic, and life-threatening disorders, as well as healthcare solutions for the general population. CLINUVEL’s innovative programs focus on the use of melanocortins for diseases of the Central Nervous System and various other organs. The patient populations in these diseases range in size from 5,000 to 45 million worldwide.
As pioneers in photomedicine and understanding the interaction of light and human biology, CLINUVEL’s research and development has led to innovative treatments for patient populations with a clinical need for systemic photoprotection, DNA repair and acute or life-threatening conditions.
Headquartered in Melbourne, Australia, CLINUVEL has operations in Europe, Singapore and the USA. For more information please go to https://www.clinuvel.com.
SCENESSE® and PRÉNUMBRA® are registered trademarks of CLINUVEL PHARMACEUTICALS LTD.
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD.
Media enquiries
Monsoon Communications
Mr Rudi Michelson, +61 411 402 737,
rudim@monsoon.com.au
Investor Enquiries
https://www.clinuvel.com/investors/contact-us
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance, or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products, the COVID-19 pandemic affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical products; competition for our products, especially SCENESSE® (afamelanotide 16mg); our ability to achieve expected safety and efficacy results through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, China and Japan of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE® which may lead to it being unable to supply its commercial markets and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; any failure to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2020 Annual Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on the forecasts and estimates is available on request. Past performance is not an indicator of future performance.
www.clinuvel.com
Level 11
535 Bourke Street
Melbourne
Victoria, Australia, 3000
T +61 3 9660 4900
F +61 3 9660 4999