First stroke patient treated with afamelanotide
| Melbourne, Australia, 07 June 2021 | ASX:
XETRA-DAX: NASDAQ INTERNATIONAL DESIGNATION: |
CUV
UR9 CLVLY |
|---|
CLINUVEL’s drug afamelanotide has been administered to a first patient diagnosed with an acute arterial ischaemic stroke (AIS) enrolled in a world’s first clinical trial (CUV801). Due to a clot formed in the higher region of the left middle cerebral artery (M2 or above), the patient suffered an acute stroke and was admitted to a specialist neurological hospital in Australia to receive treatment.
In total, six adult AIS patients will be evaluated in the Phase II CUV801 study. The study focuses on the safety and therapeutic potential of afamelanotide in patients who are ineligible for standard stroke therapy.
“The immediate aim in acute AIS treatment, is to bring back the patient’s neurological and muscular functions by improving the blood flow to the affected site of the brain,” CLINUVEL’s Clinical Operations Manager, Dr Pilar Bilbao said. “Our unambiguous aim is to develop a treatment for 70% to 80% of the stroke patients who currently have no alternative treatment”.
AFAMELANOTIDE TO TREAT ARTERIAL ISCHAEMIC STROKE (AIS)
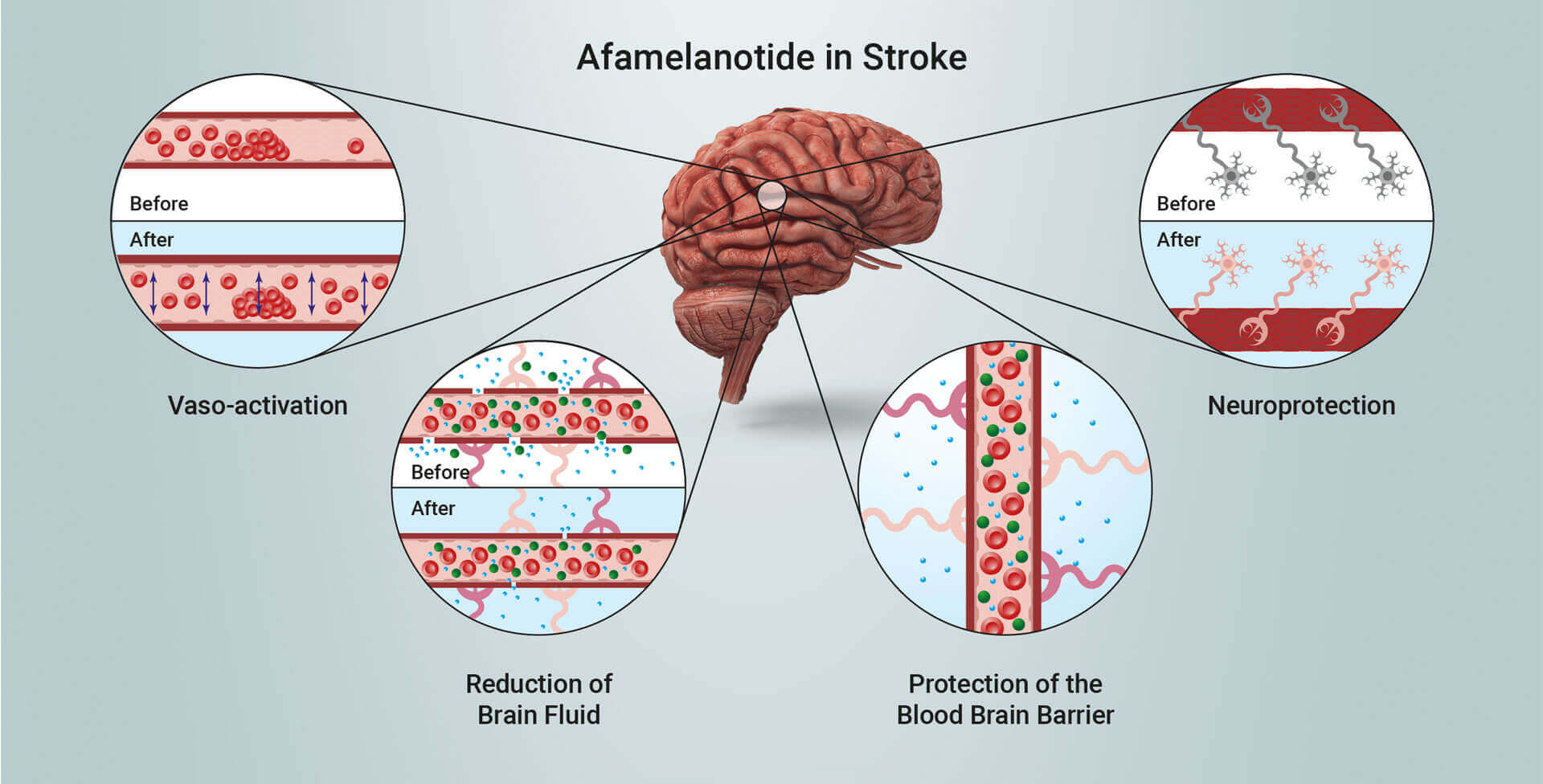
Scientific progress has demonstrated melanocortins, including afamelanotide, provide a positive effect on the central nervous system (CNS). Afamelanotide is known to offer neuroprotection and act as a potent anti-oxidative hormone. The drug possesses further therapeutic benefits, activating vessels, reducing fluid formation, protecting critical nerve and brain tissue, and restoring the blood brain barrier (BBB: a critical defence mechanism protecting the brain).
The drug therapy is expected to affect the blood flow and oxygen to deprived brain tissue.
ACUTE STROKE
Stroke is the second most common reason of death and a leading cause of disability worldwide, yet many stroke patients are unsuitable to receive the current standard of care (clot removal and clot dissolution).
Acute stroke most frequently occurs unexpectedly and without warning. The main reason for AIS is a severe constriction of, and a clot lodged within, the brain vessel. AIS leads to an immediate lack of oxygen and glucose supply, resulting in partial death of brain tissue. A stroke patient may suddenly lose consciousness, and typically will experience loss of movement of one side of the body (such as the arms, legs or face).
Following a stroke, family and bystanders usually ensure that the patient is immediately transported to the emergency department of a hospital, where a brain scan (computed tomographic angiography; CTA) and clinical examination need to confirm the diagnosis. Partial or full recovery of the patient depends on the size of the infarct (dead brain tissue) incurred, speed of treatment offered, and underlying general health.
Unfortunately, the majority of stroke patients do not receive standard therapy because it cannot be offered within the internationally accepted critical treatment window of four and a half hours, or because the clot is lodged in inaccessible parts of the brain (M2 branch and higher).
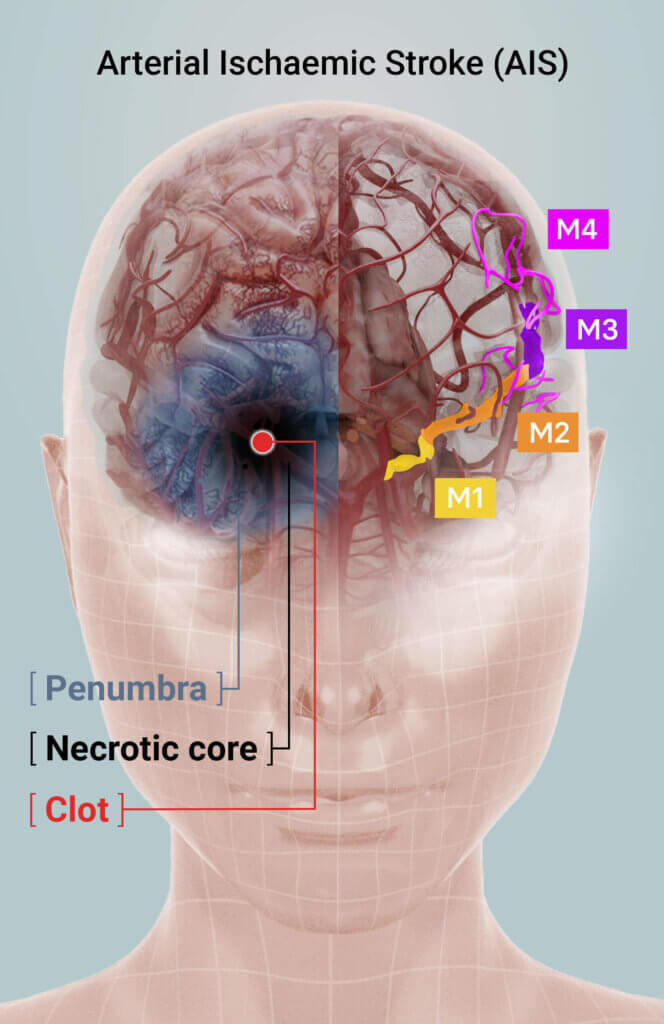
Figure 1: A sudden clot lodged in a brain vessel causes an acute stroke leading to a zone of dead brain tissue, known as the necrotic core. The area surrounding the core is at immediate risk of further tissue death and is called the penumbra: brain tissue which can be returned to normal function if immediate intervention can be offered. The right side of the image shows the middle cerebral artery (MCA) sections M1–M4.
CUV801 STUDY – AFAMELANOTIDE IN AIS
The CUV801 study is evaluating the use of afamelanotide in six patients who suffered an acute stroke, with a main focus on safety monitoring following drug administration as they are admitted to hospital.
Following multiple dosing, patients enrolled in the study are clinically assessed to detect changes or improvement in neurological functions and activities of daily living. Validated clinical tools, the Modified Rankin Scale and National Institutes of Health Stroke Scale, are used to evaluate the extent of patients’ disability.
Through a number of periodic magnetic resonance imaging (MRI) brain scans, the blood volume and flow to the affected regions of the brain are assessed, with a special attention to the core of the stroke (infarct) and penumbra.
– END –
ABOUT ACUTE STROKE
AIS accounts for approximately 85% of the 15 million strokes suffered worldwide each year. Despite its prevalence, treatment options are limited: in Europe, over 85% of AIS cases presenting to hospitals are not eligible for current standard of care treatment (thrombectomy and thrombolysis).
ABOUT AFAMELANOTIDE
Afamelanotide exhibits neuroprotective, vasoactive (acting on blood vessels), anti-oncotic (anti-swelling) and anti-oxidative effects by optimising blood flow and reducing the size of injury and fluid (oedema) formation. The drug has been shown in non-clinical models to restore the flow of blood and oxygen to the brain after stroke and reduce the extent of cerebral damage. SCENESSE® (afamelanotide 16mg) is approved in Europe, the USA and Australia for a rare light genetic metabolic disorder called erythropoietic protoporphyria (EPP). Information on SCENESSE® can be found on CLINUVEL’s website at www.clinuvel.com.
For further details on current standard of care in stroke and M2 and higher segments of the MCA, please see CLINUVEL’s October 2020 announcement of the AIS program.
ANNEX I: FOLLOWING ASX BEST PRACTICE
Name of trial
A Proof of Concept, Phase IIa, Open Label Study to Evaluate the Safety of Afamelanotide in Patients with acute Arterial Ischaemic Stroke (AIS) due to Distal [M2 and beyond] Arterial Large Vessel Occlusion (LVO) or Perforator Occlusion and who are ineligible for Intravenous Thrombolysis (IVT) or Endovascular Thrombectomy (EVT) (CUV801).
Primary endpoint
To evaluate the safety of afamelanotide in acute Arterial Ischaemic Stroke (AIS)
Secondary endpoints
Identify changes in reperfusion of the ischaemic penumbra in AIS patients, specifically the ischaemic core and or the penumbral ischaemic zone (salvageable tissue).
Assess neurological functions and activities of daily living in AIS patients.
Blinding status
Open label.
Product development status
Good Manufacturing Practice (GMP) Standard.
Treatment method and dose levels
SCENESSE® (afamelanotide) implants, total of 64mg of afamelanotide following stroke.
Number of trial subjects
Up to six AIS patients.
Subject selection criteria
To be eligible to enter the study, patients must meet the following inclusion criteria:
- Male or female subjects with a diagnosis of Arterial Ischaemic Stroke (AIS)
- Mild to moderate stroke
- No severe disability prior to stroke
- Written informed consent obtained from patient and or immediate family or carer(s) prior to study-start.
Further safety related exclusion criteria apply.
Trial location
One specialist stroke treatment centre in Melbourne, Australia.
Duration of trial
42 days
Trial standard
In compliance with Good Clinical Practice (GCP) and ICH guidelines.
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD
ABOUT CLINUVEL PHARMACEUTICALS LIMITED
CLINUVEL PHARMACEUTICALS LTD (ASX: CUV; NASDAQ INTERNATIONAL DESIGNATION ADR: CLVLY; XETRA-DAX: UR9) is a global and diversified biopharmaceutical company focused on developing and commercialising treatments for patients with genetic, metabolic, and life-threatening disorders, as well as healthcare solutions for the general population. CLINUVEL’s innovative programs focus on the use of melanocortins for diseases of the Central Nervous System and various other organs. The patient populations in these diseases range in size from 5,000 to 45 million worldwide.
CLINUVEL’s lead compound, SCENESSE® (afamelanotide 16mg), was approved by the European Commission in 2014, the US Food and Drug Administration in 2019 and the Australian Therapeutic Goods Administration in 2020 for the prevention of phototoxicity (anaphylactoid reactions and burns) in adult patients with erythropoietic protoporphyria (EPP). As pioneers in photomedicine and understanding the interaction of light and human biology, CLINUVEL’s research and development has led to innovative treatments for patient populations with a clinical need for systemic photoprotection, DNA repair and acute or life-threatening conditions.
More information on EPP can be found at http://www.epp.care. Headquartered in Melbourne, Australia, CLINUVEL has operations in Europe, Singapore and the USA. For more information please go to http://www.clinuvel.com.
SCENESSE® and PRÉNUMBRA® are registered trademarks of CLINUVEL PHARMACEUTICALS LTD.
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD
Media enquiries
Monsoon Communications
Mr Rudi Michelson, 61 411 402 737, rudim@monsoon.com.au
Investor Enquiries
https://www.clinuvel.com/investors/contact-us
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance, or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products, the COVID-19 pandemic affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical products; competition for our products, especially SCENESSE® (afamelanotide 16mg); our ability to achieve expected safety and efficacy results through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, China and Japan of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE® which may lead to it being unable to supply its commercial markets and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; any failure to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2020 Annual Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on the forecasts and estimates is available on request. Past performance is not an indicator of future performance.
www.clinuvel.com
Level 11
535 Bourke Street
Melbourne
Victoria, Australia, 3000
T +61 3 9660 4900
F +61 3 9660 4999
“Medical innovation lies at the core of our professional drive, however the current success comes from the thoughtful decisions implemented by our team”
Dr Bilbao