Revenue Growth Drives CLINUVEL’s Sixth Consecutive Annual Profit
| Melbourne, Australia, 30 August 2022 | ASX: XETRA-DAX: ADR Level 1: |
CUV UR9 CLVLY |
An Investor Webinar will be held today (30 August) at 18:00-18:30 AEST (10:00-10:30 CEST) – see details below.
Key Highlights, Year Ending 30 June 2022
| Consolidated Entity | 30 June 2022 | 30 June 2021 |
|---|---|---|
| Total Revenues, Interest and Other Income | $66,987 | $48,451 |
| Total Expenses | $32,667 | $22,738 |
| Net Profit before income tax expense | $34,321 | $25,713 |
| Profit after income tax expense | $20,876 | $24,728 |
| Cash and Cash Equivalents | $121,509 | $82,691 |
| Basic Earnings per Share | $0.42 | $0.50 |
| Net Tangible Assets backing per Share | $2.50 | $1.91 |
| Dividend distribution per Share | $0.04 | $0.025 |
| All figures are reported in Australian dollars. Refer to the Appendix 4E and Annual Report released to the Australian Securities Exchange for details.1 Figures in A$ ‘000s. Refer to the Appendix 4E Preliminary Final Report released to the Australian Securities Exchange for details | ||
CLINUVEL today announced its sixth consecutive annual net profit, driven by revenues of $66.987 million, a 38% increase. For the year ending 30 June 2022 (FY2022), the Group has reported profit after income tax expense (NPAT) of $20.876 million and profit before income tax expense (PBIT) of $34.321 million, as disclosed in its Appendix 4E and Annual Report. The Group also reported a non-IFRS measure of adjusted net profit before tax of $39.837 million, which adjusts for certain material non-cash items that do not impact the Group’s cash position.
“CLINUVEL’s commercial operations are scaling up to meet treatment demand worldwide, while the Group is pursuing R&D projects which aim to add value over the long-term,” CLINUVEL’s Chief Financial Officer, Mr Darren Keamy said. “Our FY2022 results show a fundamentally strong business to date, allowing us to invest for future growth.
“Improved cash inflows this year have further bolstered the Company’s cash reserves enabling us to continue the implementation of a growth strategy in the face of difficult economic headwinds. The strong cash position has also allowed the Board to declare an increase in dividend this year, most of all recognising the loyalty and patience of long-term shareholders. We remain focused to translate our technology to the benefit of patients and specialised populations, particularly those at highest risk of light-induced damage and skin cancer,” Mr Keamy said.
Six Years of Revenue Growth and Profit
CLINUVEL remains one of the few ASX-listed bio-pharmaceutical sciences companies to generate a profit. In FY2022 the Company delivered its sixth consecutive year of positive cashflow, revenues and profit, while maintaining control of operating expenses to facilitate growth and expansion.

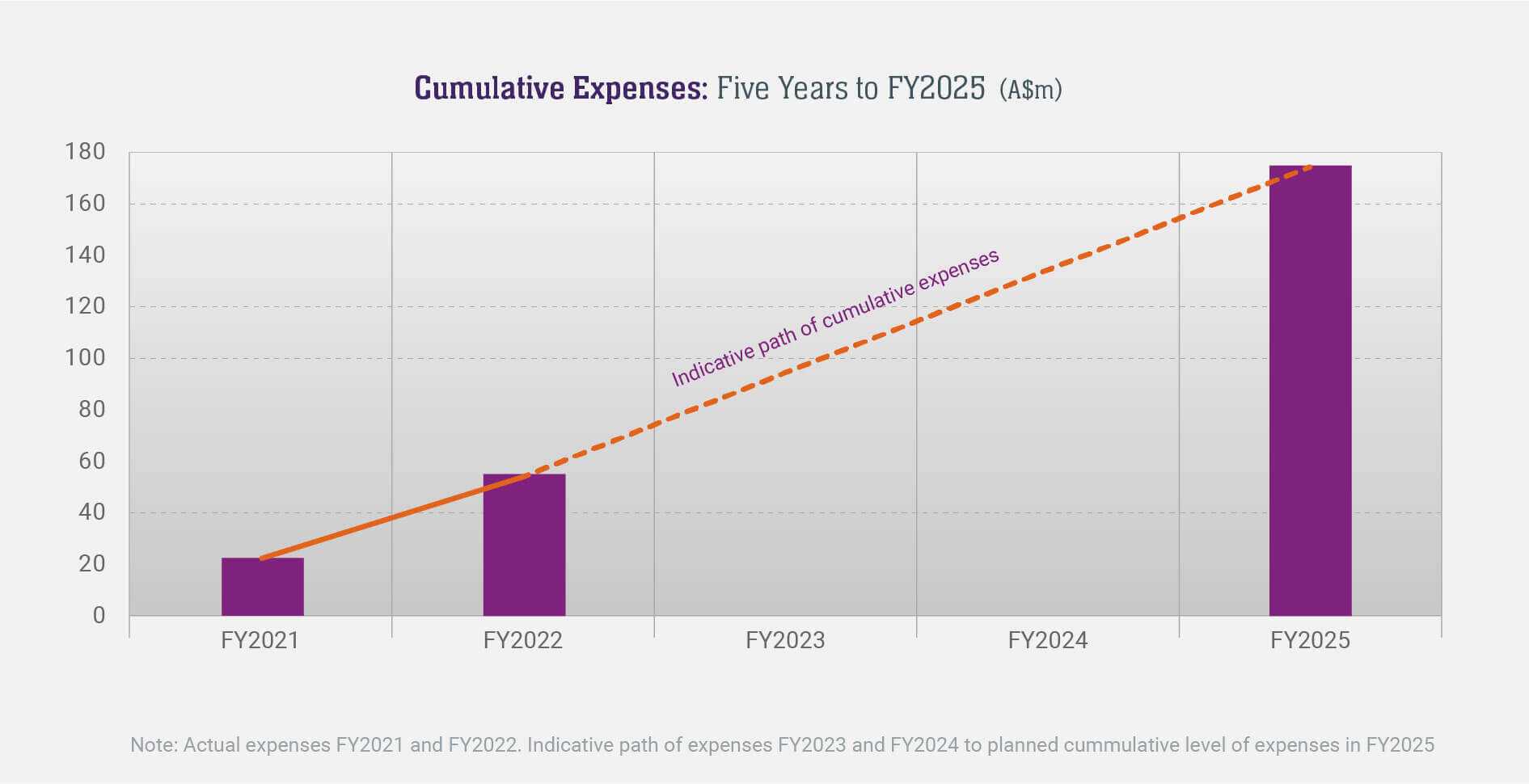
The Group has outlined a clear long-term growth strategy reinvesting a large percentage of proceeds into new development. The expansion strategy is enabled by the ongoing increase of cash inflows from commercial operations. The accumulated cash reserves increased by $38.818 million (47%) during FY2022 to stand at $121.509 million as of 30 June 2022. CLINUVEL has committed to expenditures of $175 million over the five years to 30 June 2025 to achieve its growth and expansion objectives and, based on expenses in FY2021 and FY2022, is on track to achieve this projection.
More information on the Company’s strategy can be found at www.clinuvel.com.
Increase in Annual Dividend
Following the financial results for the year ending 30 June 2022, the CLINUVEL Board has declared an increase to its full-year dividend distribution to $0.04 per ordinary share fully franked, up from 2.5 cents per share unfranked for the full year to 30 June 2021. This is the fifth consecutive dividend declared by the Group. Subject to sufficient cash reserves, the key dates for the dividend are:
Ex-dividend date: 06 September 2022;
Record date: 07 September 2022; and
Payment date: 21 September 2022.
Dividends are available to Australian and overseas registered shareholders, including holders of CLINUVEL’s Level 1, American Depository Receipts. Prior to the record date, shareholders are encouraged to confirm their personal shareholder information, including payment election information, with the share registrar.
Clinuvel Briefing
CLINUVEL will host an investor and analyst webinar at 18:00 AEST today to review the results of the FY2022. Participants can register using the link below:
Investor Zoom Webinar 18:00-18:30 AEST (10:00-10:30 CEST) today (30 August)
To participate, please register using this link:
https://us06web.zoom.us/webinar/register/WN_YCmt2BpiSlingw14CJSLXg
Questions may be tabled as you register or during the webinar
CLINUVEL’s Appendix 4E and Annual Report is available on the Company’s website, www.clinuvel.com.
– End –
About CLINUVEL PHARMACEUTICALS LIMITED
CLINUVEL (ASX: CUV; ADR LEVEL 1: CLVLY; XETRA-DAX: UR9) is a global specialty pharmaceutical group focused on developing and commercialising treatments for patients with genetic, metabolic, systemic, and life-threatening, acute disorders, as well as healthcare solutions for the general population. As pioneers in photomedicine and the family of melanocortin peptides, CLINUVEL’s research and development has led to innovative treatments for patient populations with a clinical need for systemic photoprotection, DNA repair, repigmentation and acute or life-threatening conditions who lack alternatives.
CLINUVEL’s lead therapy, SCENESSE® (afamelanotide 16mg), is approved for commercial distribution in Europe, the USA, Israel and Australia as the world’s first systemic photoprotective drug for the prevention of phototoxicity (anaphylactoid reactions and burns) in adult patients with erythropoietic protoporphyria (EPP). Headquartered in Melbourne, Australia, CLINUVEL has operations in Europe, Singapore and the USA. For more information, please go to https://www.clinuvel.com.
SCENESSE®, PRÉNUMBRA®, and NEURACTHEL® are registered trademarks of CLINUVEL.
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD
Media Enquiries
Monsoon Communications
Mr Rudi Michelson, 61 411 402 737, rudim@monsoon.com.au
Head of Investor Relations
Mr Malcolm Bull, CLINUVEL PHARMACEUTICALS LTD
Investor Enquiries
https://www.clinuvel.com/investors/contact-us
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products; the COVID-19 pandemic and/or other world, regional or national events affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical products; competition for our products, especially SCENESSE® (afamelanotide 16mg), PRÉNUMBRA® or NEURACTHEL®; our ability to achieve expected safety and efficacy results in a timely manner through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, Israel, China and Japan of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE®, PRÉNUMBRA® or NEURACTHEL® which may lead to it being unable to supply its commercial markets and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology and consumer based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; our ability to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2021 Annual Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on preliminary and uncertain forecasts and estimates is available on request, whereby it is stated that past performance is not an indicator of future performance.
www.clinuvel.com
Level 11, 535 Bourke Street, Melbourne, Victoria, Australia, 3000, T +61 3 9660 4900, F +61 3 9660 4909