CLINUVEL progresses vitiligo treatment program
| Melbourne, Australia, 10 May 2022 | ASX: XETRA-DAX: ADR Level 1: |
CUV UR9 CLVLY |
A new potential therapy for the pigment loss disorder vitiligo will undergo further studies in North America this year. The drug afamelanotide – approved by US and European regulators for a rare light intolerance disorder – is being evaluated by Australian company CLINUVEL as a treatment for vitiligo patients with darker skin types.
Vitiligo causes the progressive loss of skin pigment in lesions (patches) across the body and affects an estimated 45 million individuals worldwide. Pigment loss caused by vitiligo is most pronounced in patients with darker skin types who report the greatest impact of the disease on their quality of life. Treatment options remain limited, with no treatments for repigmentation of vitiligo currently approved by the US Food and Drug Administration (FDA).
“Many vitiligo patients must grapple with a sense of lost identity as their skin visibly and inexplicably changes, forcing them to withdraw from society and having a severe impact on their mental health and quality of life,” CLINUVEL’s Director of North American Operations, Dr Linda Teng said.
“Clinical trials of afamelanotide to date have shown the drug can assist in repigmenting vitiligo patients’ skin, with the most promising results in darker skinned patients. Therefore we have chosen to focus on this patient group, with the highest unmet need, as our clinical program progresses,” Dr Teng said.
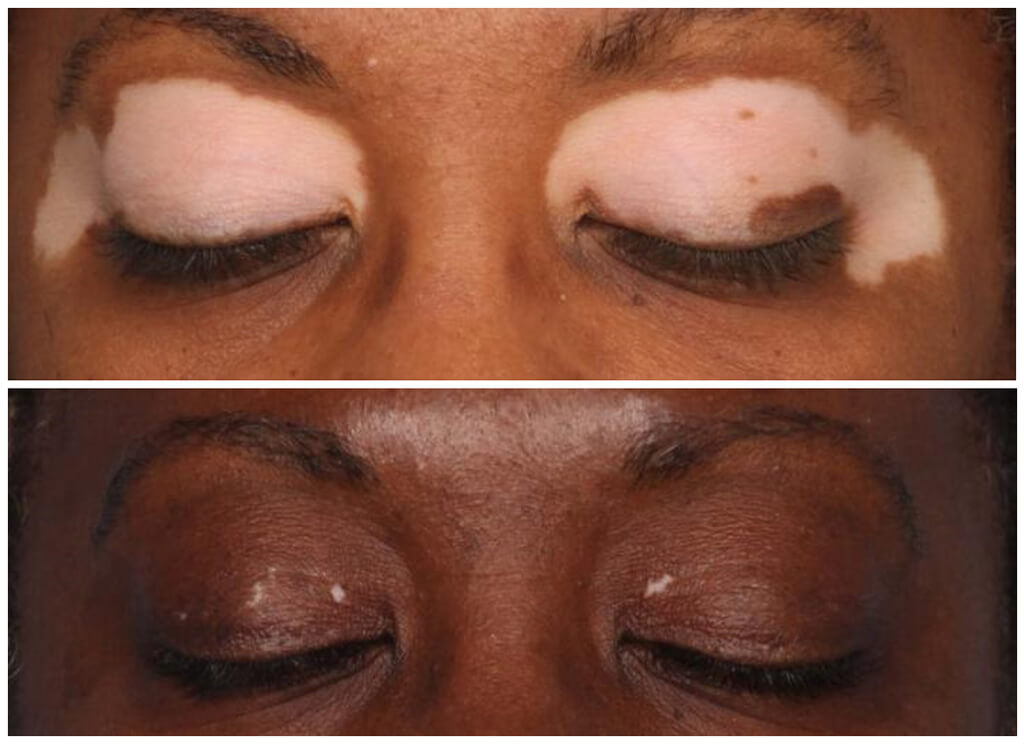
Afamelanotide as repigmentation therapy
One of a new class of drugs known as melanocortins, afamelanotide activates melanin, the pigment in skin. The drug is also understood to play a role in maintaining the overall health of skin including melanocytes, the skin cells responsible for melanin production.
“Many experimental vitiligo treatment approaches to date have been ineffective, inconvenient, or cause unpleasant side effects, often seeking to bluntly suppress the immune system to allow for repigmentation,” Dr Teng said. “Afamelanotide treatment is expected to help regulate immune and inflammatory responses in the skin while restoring patients’ pigmentary function: stimulating melanocytes to migrate to affected skin and produce melanin. Preliminary results should be available late this year, with full results in 2023.”
CLINUVEL’s initial studies in vitiligo patients showed that afamelanotide could repigment skin in combination with a light-based therapy, with nearly 100 patients receiving treatment to date and the safety profile of the drug maintained.1,2 The program will now focus on afamelanotide as a monotherapy after approval of the CUV104 study was received from the Institutional Review Board (IRB) at the specialist medical centre conducting the study.
CUV104 will commence in North America during northern summer months. Adult vitiligo patients will receive multiple doses of afamelanotide with expert physicians monitoring repigmentation and safety for six months. The impact of treatment on the patients’ quality of life will be captured using validated clinical tools.
Up to six patients will be enrolled in CUV104, sufficient to meet the statistical needs of a pilot study, provide feedback on protocol designs for larger studies of afamelanotide as a monotherapy, and complete quickly in a clinical environment where COVID-19 restrictions have impacted study enrolment rates.
In total, over 11,000 doses of afamelanotide have been administered to over 1,600 individuals worldwide across clinical, special access and post-authorisation programs.2
– End –
Media Enquiries: Monsoon Communications, Mr Rudi Michelson, 61 411 402 737, rudim@monsoon.com.au
1 Results from these studies have been published in peer-reviewed journals:
- Lim, H. W. et al. (2015). Afamelanotide and Narrowband UV-B Phototherapy for the Treatment of Vitiligo: A Randomized Multicenter Trial. JAMA Dermatology, 151(1), 42.
- Toh, J. et al. (2020). Afamelanotide implants and narrow-band ultraviolet B phototherapy for the treatment of nonsegmental vitiligo in Asians. Journal of the American Academy of Dermatology, 82(6), 1517–1519.
2 SCENESSE® (afamelanotide 16mg) is approved in the European Union and Australia as an orphan medicinal product for the prevention of phototoxicity in adult patients with erythropoietic protoporphyria (EPP). SCENESSE® is approved in the USA to increase “pain- free” light exposure in adult EPP patients with a history of phototoxicity. Information on the product can be found on CLINUVEL’s website at www.clinuvel.com.
About CLINUVEL PHARMACEUTICALS LIMITED
CLINUVEL (ASX: CUV; ADR LEVEL 1: CLVLY; XETRA-DAX: UR9) is a global specialty pharmaceutical group focused on developing and commercialising treatments for patients with genetic, metabolic, systemic, and life-threatening, acute disorders, as well as healthcare solutions for the general population. As pioneers in photomedicine and the family of melanocortin peptides, CLINUVEL’s research and development has led to innovative treatments for patient populations with a clinical need for systemic photoprotection, DNA repair, repigmentation and acute or life-threatening conditions who lack alternatives.
CLINUVEL’s lead therapy, SCENESSE® (afamelanotide 16mg), is approved for commercial distribution in Europe, the USA, Israel and Australia as the world’s first systemic photoprotective drug for the prevention of phototoxicity (anaphylactoid reactions and burns) in adult patients with erythropoietic protoporphyria (EPP). Headquartered in Melbourne, Australia, CLINUVEL has operations in Europe, Singapore and the USA. For more information, please go to https://www.clinuvel.com.
SCENESSE®, PRÉNUMBRA®, and NEURACTHEL® are registered trademarks of CLINUVEL.
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD
Head of Investor Relations
Mr Malcolm Bull, CLINUVEL PHARMACEUTICALS LTD
Investor Enquiries
https://www.clinuvel.com/investors/contact-us
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products; the COVID-19 pandemic and/or other world, regional or national events affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical products; competition for our products, especially SCENESSE® (afamelanotide 16mg), PRÉNUMBRA® or NEURACTHEL®; our ability to achieve expected safety and efficacy results in a timely manner through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, Israel, China and Japan of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE®, PRÉNUMBRA® or NEURACTHEL® which may lead to it being unable to supply its commercial markets and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology and consumer based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; our ability to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2021 Annual Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on preliminary and uncertain forecasts and estimates is available on request, whereby it is stated that past performance is not an indicator of future performance.
www.clinuvel.com
Level 11, 535 Bourke Street, Melbourne, Victoria, Australia, 3000, T +61 3 9660 4900, F +61 3 9660 4909