Appendix 4C
Melbourne, Australia, 30 April 2019
CLINUVEL PHARMACEUTICALS LTD, a global biopharmaceutical company focused on developing and delivering treatments for patients with a range of severe genetic and skin disorders, today announced its Appendix 4C – Quarterly Cashflow Report for the period 01 January to 31 March 2019. All figures are rounded and reported in Australian dollars.
Cash Receipts for the quarter were $5,898,000, an increase of 126% on the December quarter 2018 ($2,608,000) and up 70% compared to the March quarter 2018 ($3,480,000). Financial year-to-date cash receipts to March 2019 of $19,211,000 were 44% higher than for the prior corresponding period to March 2018.
All Cash Receipts were earned from the SCENESSE® (afamelanotide 16mg)¹ treatment provided in the European Union and Switzerland for patients with the rare metabolic disorder erythropoietic protoporphyria (EPP). Unit sales of SCENESSE® are generally lower in the winter months due to the lower intensity of ambient light. This results in a mean lower clinical demand in most countries receiving SCENESSE® during this period. Conversely, stronger clinical demand in the northern hemisphere spring and summer positively impacts Cash Receipts in the June and September quarters.
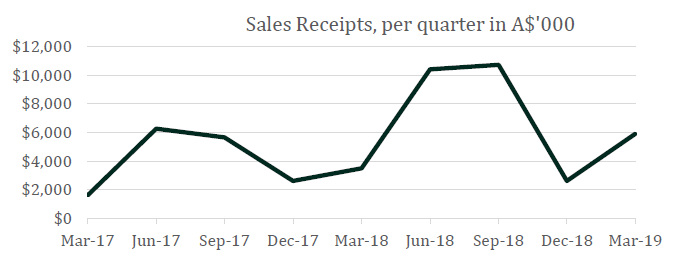
The financial year-to-date cash receipt result reflects the growth in the commercial distribution program in Europe in terms of more EPP centres willing to prescribe and more patients seeking treatment and alleviating the impact of sales fluctuations on company cashflow.
Net Operating Payments for the March quarter 2019 were $3,584,000 compared to $3,331,000 in the December quarter 2018 and $3,302,000 in the March quarter 2018. The 7% increase from the December quarter 2018 is due to increases in product manufacturing expenditures to prepare for an expected increase in sales as we progress into the spring and summer seasons in the northern hemisphere. Taking into account the rise in product manufacturing expenditures, the overall modest difference in these cash outflows across the comparative periods reflects the stable operating cost base of the business and cautious cost management.
Net Cash from Operations was positive by $2,516,000 in the March quarter 2019. Net Cash from Operations for the nine months to 31 March 2019 was $9,764,000 compared to $4,253,000 in the same period to 31 March 2018. As a result of the positive cashflow in the quarter, the Cash Balance as at 31 March 2019 increased to $44,975,000, compared to $42,826,000 as at 31 December 2018 and $28,915,000 as at 31 March 2018.
During the March quarter 2019, CLINUVEL remained focused on the management of its European business and responding to the US Food and Drug Administration (FDA) on its New Drug Application for approval to distribute the first-in-class treatment, SCENESSE® to EPP patients in the USA. The FDA is currently assessing CLINUVEL’s application under a Priority Review with an advised date (PDUFA) of 8 July 2019 for its decision.
COMMENTARY
“Whilst our quarterly cashflow results are influenced by the seasonality of demand of European patients, our volume growth year on year is trending positively”, CLINUVEL’s Chief Financial Officer Mr Darren Keamy said. “This, coupled with consistent cost management, has driven the positive cashflow outcome and improved cash balance in the March quarter of 2019. Our European office has prepared itself for the impending spring and summer months where, as seen in recent years, sales orders for SCENESSE® tend to increase.”
– End –
1 SCENESSE® (afamelanotide16mg) is approved in Europe as an orphan medicinal product for the prevention of phototoxicity in adult patients with EPP. Information on the product can be found on CLINUVEL’s website at www.clinuvel.com.