Melanocortins

About Melanocortins
CLINUVEL’s pharmaceutical development to date has focused on melanocortins, a group of small protein hormones that develop from the gene product proopiomelanocortin (POMC).
These hormones modulate physiological activity in the body by binding with specific melanocortin receptors, of which there are currently five identified: MC1R—MC5R. These receptors are widespread within the human body and present in virtually every organ, making the effects of the melanocortins widespread and varied.
Known physiological activity influenced by melanocortins includes pigmentation, inflammation, energy homeostasis, appetite and sexual function. Melanocortins expressed in the brain, or those which cross the blood-brain barrier and bind with MC3R and MC4R (the third and fourth melanocortin receptors), are thought to play a fundamental role in feeding and body weight. Melanocortin hormones are also expressed in peripheral tissues including the duodenum, kidneys, liver, ovaries, placenta, skin and testes, where they can have a more localised response.
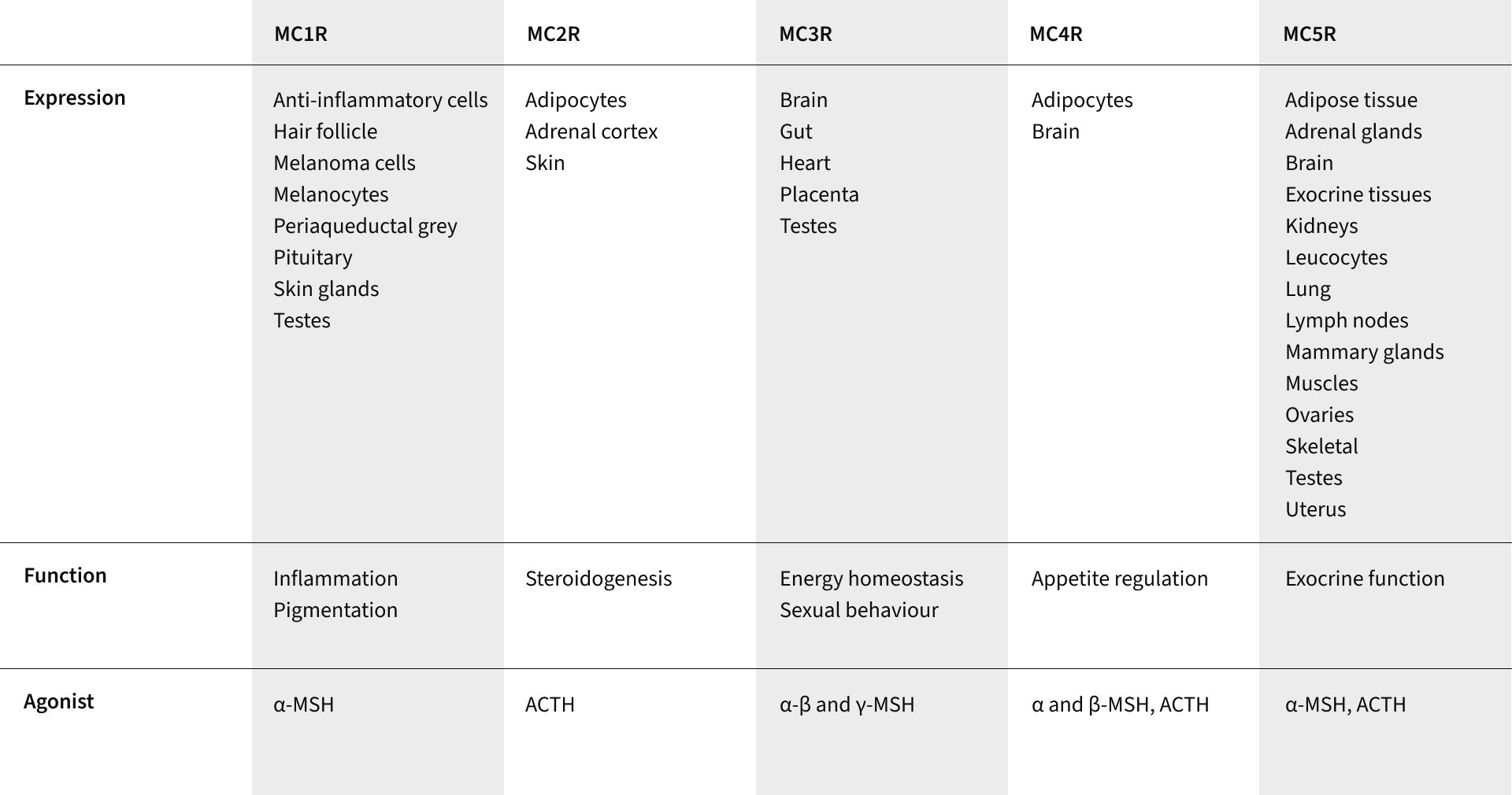
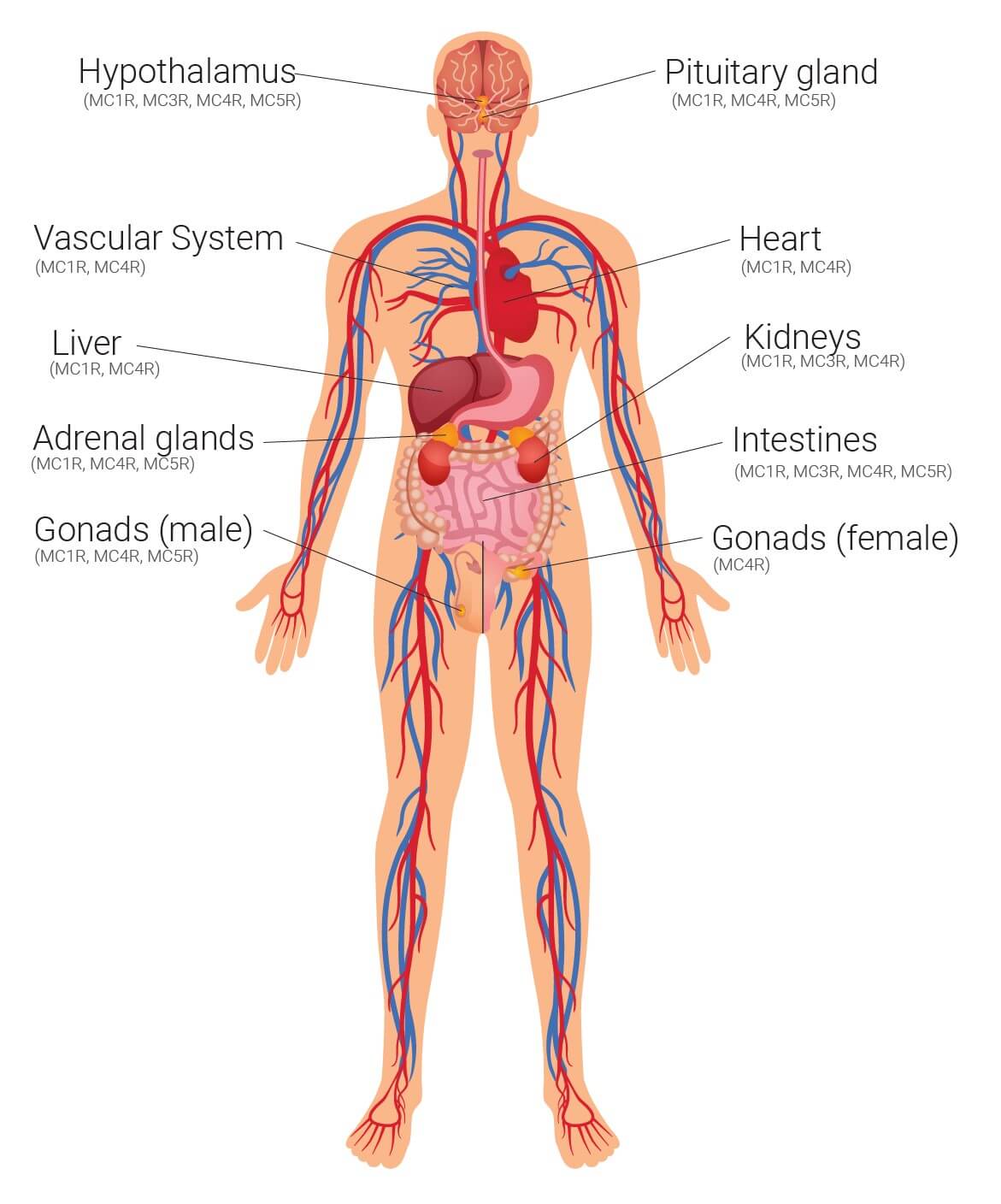
Melanocortin receptors identified throughout the body
About Alpha-MSH
Alpha-melanocyte stimulating hormone (α-MSH) is a protein hormone produced in the body. It is part of a family of peptides known as melanocortins, all of which are cleaved from the precursor polypeptide proopiomelanocortin (POMC) and bind to specific melanocortin receptors throughout the body. α-MSH consists of 13 amino acids, with the sequence Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val.
Of the five known melanocortin receptors, α-MSH binds with various affinities to MC1R, MC3R, MC4R and MC5R. When bound to each of these receptors it is believed to have roles in: regulating pigmentation and inflammation, DNA repair, energy homeostasis, sexual behaviour, appetite regulation and exocrine function.
Alpha-melanocyte stimulating hormone in the skin
It is α-MSH’s role In stimulating melanin production (melanogenesis) in the skin which is arguably best understood. In the skin, α-MSH is produced by epidermal skin cells (keratinocytes and, less commonly, melanocytes and Langerhans cells) as a protective response to damage caused by ultraviolet radiation (UVR).
Alpha-MSH molecules then bind with the MC1R on melanocytes (pigment producing cells) to activate the synthesis of black-brown melanin, called eumelanin. Following this process, eumelanin granules are synthesised within intracellular packages called melanosomes which are then transported to the ends of tentacle-like projections in the cell, called dendrites.
The tips of these dendrites touch and release melanosomes into adjacent keratinocytes. The melanosomes cluster around keratinocyte nuclei to form a pigmented, protective barrier. The eumelanin within melanosomes shields cells from further UVR damage by absorbing, reflecting and refracting light.
In addition to activating melanin, α-MSH is known to have several other roles in the skin, although the exact mechanisms are not fully understood. Recent research has shown that α-MSH enhances the repair of DNA damage, a process known as nucleotide excision repair (NER), and reduces the generation of free radicals following UVR exposure. Both factors reduce the overall damage caused by UVR, thus reducing the risk factors for certain skin cancers. However some people with fairer skin types have reduced MC1R activity, meaning the response when α-MSH binds to the MC1R is impaired or absent. Alpha-MSH is also known to play a role in inhibiting both the expression and activity of inflammatory cytokines in the skin.
Published research
McNeil, M. M., Nahhas, A. F., Braunberger, T. L., Hamzavi, I. H. Afamelanotide in the Treatment of Dermatologic Disease. Skin Ther Lett. 2018;23:6-10.
Rodrigues, M., Ezzedine, K., Hamzavi, I., Pandya, A. G., Harris, J. E., Vitiligo Working Group. Current and emerging treatments for vitiligo. Journal of the American Academy of Dermatology. 2017;77(1):17-29.
Minder, El, Barman-Aksoezen, J, Schneider-Yin, X. Pharmacokinetics and pharmacodynamics of afamelanotide and its clinical use in treating dermatologic disorders. Clinical Pharmacokinetics. 2017;56(8):815-823.
Lane, AM, McKay, JT, Bonkovsky, HL. Advances in the management of erythropoietic protoporphyria–role of afamelanotide. The application of clinical genetics. 2016;9:179.
Minder E, Schneider-Yin X. Afamelanotide (CUV1647) in dermal phototoxicity of erythropoietic protoporphyria. Expert review of clinical pharmacology. 2015;8(1):43-53.
Lim HW, Grimes PE, Agbai O, et al. Afamelanotide and Narrowband UV-B Phototherapy for the Treatment of Vitiligo. JAMA dermatology. 2015;151(1):42-50.
Langendonk J, Balwani M, Anderson K et al. Afamelanotide for Erythropoietic Protoporphria. New England Journal of Medicine. 2015;373(1):48-59.
Biolcati G, Marchesini E, Sorge F, Barbieri L, Schneider‐Yin X, Minder E. Long-term observational study of afamelanotide in 115 patients with erythropoietic protoporphyria. British Journal of Dermatology. 2015;172(6):1601-1612.
Bohm M, Ehrchen J, Luger TA. Beneficial effects of the melanocortin analogue Nle(4) -d-Phe(7) -α-MSH in acne vulgaris. Journal of the European Academy of Dermatology and Venereology. 2014;28(1):108-111.
Biolcati G, Aurizi C, Barbieri L, Cialfi S, Screpanti I, Talora C. Efficacy of the melanocortin analogue Nle4-D-Phe7-α-melanocyte-stimulating hormone in the treatment of patients with Hailey–Hailey disease. Clinical and experimental dermatology. 2014;39(2):168-175.
Fabrikant J, Touloei K, Brown SM. A Review and Update on Melanocyte Stimulating Hormone Therapy: Afamelanotide. Journal of drugs in dermatology: JDD. 2013;12(7):775.
Haylett AK, Nie Z, Brownrigg M, Taylor R, Rhodes L. Systemic photoprotection in solar urticaria with α-melanocyte stimulating hormone analogue [Nle(4) -D-Phe(7) ]-α-MSH. British Journal of Dermatology. 2011;164(2):407-414.
Minder, EI. Afamelanotide melanocortin MC1 receptor agonist photoprotective agent. Drugs of the Future. 2010;35(5):365-372.
Harms, J, Lautenschlager S, Minder CE, Minder EI. An α-melanocyte–stimulating hormone analogue in erythropoietic protoporphyria. New England Journal of Medicine. 2009;360(3):306-307.
Harms JH, Lautenschlager S, Minder CE, Minder EI. Mitigating photosensitivity of erythropoietic protoporphyria patients by an agonistic analog of alpha-melanocyte stimulating hormone. Photochemistry and photobiology. 2009;85(6):1434-1439.
Hadley ME, Dorr RT. Melanocortin peptide therapeutics: historical milestones, clinical studies and commercialization. Peptides. 2006;27(4):921-930.
Fitzgerald LM, Fryer JL, Dwyer T, Humphrey SM. Effect of MELANOTAN®,[Nle4, D-Phe7]-α-MSH, on melanin synthesis in humans with MC1R variant alleles. Peptides. 2006;27(2):388-394.
Barnetson R, Ooi TK, Zhuang L, et al. [Nle4-D-Phe7]-alpha-Melanocyte-Stimulating Hormone Significantly Increased Pigmentation and Decreased UV Damage in Fair-Skinned Caucasian Volunteers. Journal of investigative dermatology. 2006;126(8):1869-1878.