CLINUVEL Communiqué II
Dear shareholders, friends
We welcome many new shareholders, especially the large number of German speaking investors who have discovered CLINUVEL’s story the past three months; of course, we will continue to publish our News Communiqués in German. For new readers, the News Communiqués are a continuum of updates on specific and relevant regulatory, clinical, financial and market topics, noting some corporate issues remain confidential to guard the Company from competitive pressure. In a novel format, Strategic Update II – scheduled to be released after Easter and a sequel to Strategic Update I, 29 October 2020 – will systematically discuss the progress of the Company per division, its R&D program, and other relevant matters.
Since the release of News Communiqué I on 25 January, we pause and look back at an eventful first quarter, one dominated by the roll out of four approved COVID vaccinations. In March, the European Medicines Agency (EMA) accelerated the approval of a fourth anti-viral from Janssen, allowing for a single shot vaccination. Without expressing preference for any of the four available remedies, Israeli data have shown that the Pfizer one seems to be 97% effective.
Headlines were made in February, as we witnessed how Gamestop, a stock under short attack from syndicated hedge funds, had attracted speculative attention of retail shareholders as they openly rallied against the three hedge funds which had shorted the stock. On market buying support was coordinated through Reddit. As the stock price catapulted in late January, taking the company from a US$3 billion to a US$22 billion valuation, a question arose as to the role of the clearance house Robinhood and its connections with its clients Melvin Capital and Point72. As the dust settled, the fundamental valuation of Gamestop, a retail gaming company, became the focal point.
I view the Reddit phenomenon as one which illustrated the aggregate power of US retail shareholders wielding influence when it mattered. I do believe we will see more frenzies in securities as the world continues to connect faster and spread corporate news flow as it emerges.
In March, we learned how US banks and markets reeled from the default by Archegos Capital Management. The larger US investment banks had allowed the family office to take on billions of dollars of exposure to volatile equities through swap contracts, until margin calls were triggered by a plunge in the markets. This shows how the bet on one security going awry, in this case ViacomCBS shares, can instantly lead to an international cascade of proportion. As two of the largest investment banks on the street had liquidated up to US$19 billion in US and Chinese positions on one day, many other portfolio managers needed to respond by block sale at discounted prices. Keeping in mind this event is barely one month after the Greensill sage uncovered US$3 billion in exposure to Credit Suisse, leaving investors to guess how this one could have unravelled. Archegos reminds of the 1998 hedge fund LTCM debacle when the US government had no choice than to bail out the fund. At the core, leveraged balance sheets and unfettered lending by banks underlie the cascades unfolding when margin calls and breach of covenants are no longer avoidable, the relentless belief in turn of fortunes in leveraged bets on the markets recur time and time again. Portfolio managers eventually rebalance their holdings irrespective of corporate performance, and shareholders pay the price.
In scanning the world’s macro developments, we constantly rethink the course of CLINUVEL, and at present I hold a grim view as to the long-term impact on western economies coming from the rising tensions between China and the US, Europe and Australia. The discussions in Anchorage leave little to guess about the direction of these opposing political views on climate change and trade relationships. While we witnessed two posturing camps, I foresee little change near term. The ecopolitical tensions are further inflamed with Moscow having recalled its ambassador to the US, as the bilateral decisions are called – as “spiralling towards a dead end”.
For those who follow us regularly, I share that as part of the deliberations among CLINUVEL’s board of directors, macroeconomic issues such as trade imbalance between China and US-Europe, and the ongoing tensions in Asia-Pacific and South China Sea gain our attention. Global events shape our thinking about CLINUVEL, and navigating a listed company through many obstacles, I believe it is expected from us to deliberate the near-term impact of the macro shifts we are witnessing and specifically the possible impact on our business and supply chains.
Supply and distribution related to healthcare poses questions, since fair allocation of resources per country has now become a prevailing issue and pharma deals with governments are seen as putting certain nations at disadvantage. The latest national spats in Europe over the release of vaccines and fights to protect intellectual property in the quest for a global public good do not bode well for those who believe in globalisation as a driving economic force. I believe we are swiftly entering a “détente” where self-sufficiency will dominate our political and economic decisions.
Switching to the US, one cannot help but applaud the Congress’ bill for a US$1.9 trillion stimulus package passed on 11 March, the sixth COVID Relief Bill passed. This historical record to boost the economy needs to be put in context of the overall economic stimulus since early 2020 totalling more than US$5 trillion, unimaginable 18 months ago.
Since President Biden’s inauguration there is no news from Capitol Hill on its stance on pharmaceutical drug pricing. The latest news is that the Biden administration has shelved plans to reform the Medicare Part D and current rebates for Medicaid managed care until possible as far as 2023. We refer to the February 2019 News Communiqué for the discussion on the most favourable nations model that was to be implemented by the Trump administration and that has now received renewed attention from Vice President Kamala Harris.
At present, the discussion in democratic corners of the Administration aims to find ways to restrain list prices while benefiting the patient. However, pharmaceutical pricing is only a small part of the perceived problem. The deeper issues lie with the insurance system itself. US patients lacking insurance, and those who entered plans with high deductibles and coinsurance, need the most urgent political help. The lowering of drug prices may have very little bearing on the out-of-pocket cost, depending on how the insurance companies adjust to possible future cost-sharing arrangements. For now, we are not optimistic that the US drug reimbursement system can be overhauled since too many actors and intermediaries play a role in prescription drugs.
In CLINUVEL’s case, the direct distribution model to hospitals, the lack of availability in US pharmacy chains and well-defined patient population requiring Prior Authorization gives insurers much control over the drug price and patients receiving treatment.
Switching attention, as discussed in past News Communiqués (see July and September 2020), we remain much focussed on interest rates and inflation. With anticipation, we await the next quarter to watch how markets react to creeping inflation, negative US yields, and record-high valuations in tech stocks and specific market sectors. Nevertheless, there seems to be a widespread belief that economic recovery may be faster than predicted in December 2020 with growth expected in value equities, value-oriented investments and real estate, but a slow-down in fixed income.
With concern we follow the economic debate on the possibility of interest rate hikes as an unlikely factor to hold back economic growth for the next few years, whereby the value of cash remains a hot point of contention. As CLINUVEL grows and is compelled to make investment decisions to prolong its pipeline, these parameters prompt continuous adjustments to our plans.
We have entered a new year, one brimmed with optimism and hanging our hopes on the reversal of global health and the restart of our economies
For those who follow us regularly, I share that as part of the deliberations among CLINUVEL’s board of directors, macroeconomic issues such as trade imbalance between China and US-Europe, and the ongoing tensions in Asia-Pacific and South China Sea gain our attention.
At present, the discussion in democratic corners of the Administration aims to find ways to restrain list prices while benefiting the patient.
US Distribution
Dr Linda Teng, Director North American Operations
Although most US states are operating on a limited scale, our distribution team is making better than expected progress in current times.
We see a steady increase in US insurers partaking in the SCENESSE® (afamelanotide 16mg) treatment. On a daily basis our teams are managing the administrative demands from the submissions for “Prior Authorization” by each individual EPP patient, assisting Specialty Centers whenever they encounter delays and difficulties with insurers. Under strict guidance of data protection regulations and the Health Insurance Portability and Accountability Act (HIPAA) of 1996, our team members can be of direct help without compromising personal and medical data. This balancing act requires documentation, databases and frequent auditing.
The unique J-code was assigned to SCENESSE® on 01 January 2021, and we have since seen a number of changes occurring.
First, in some cases Prior Authorization is now being granted per annum, taking away the red tape of requesting Prior Authorization prior to each implant. Some insurance groups started setting PA for the duration of five years.
Second, we observe that Centers have freed-up their administrative burden to spend more clinical time with patients.
Lastly, the prescribing Centers are getting better and faster in processing patients’ administrative duties, having come through the first learning phase of managing and handling SCENESSE®.
The next stage is for our teams to secure a treatment-specific Current Procedural Terminology (CPT®)-code. Although this usually takes more than two years, we have managed to speed this up by one year.
A CPT® -code allows a set of codes, descriptions, and guidelines intended to describe medical procedures and services for processing claims, conducting research, evaluating healthcare utilisation, and developing medical guidelines and other forms of healthcare documentation.
The CPT® code system was adopted as part of the Centers for Medicare & Medicaid Services (CMS), Healthcare Common Procedure Coding System (HCPCS). The HCPCS code set is divided into two principal subsystems: (1) Level I of the HCPCS, which comprised the CPT® and (2) Level II of the HCPCS. Level I CPT® codes are five-digit numerical codes used to describe medical procedures or services performed by healthcare professionals.
Ultimately, the American Medical Association (AMA) is responsible for the additions, deletions, revisions, and annual updates of the CPT® code system. As part of HIPAA, the Department of Health and Human Services designated CPT® and HCPCS as the national standards for electronic transaction of healthcare information.
Every medical, diagnostic, or surgical procedure or service has a CPT® code assigned to it. These are used by healthcare professionals, hospitals, clinics, and insurance companies to identify healthcare services and procedures in federal programs and through commercial and/or private insurers. CPT® codes are also used by insurers to determine the amount a healthcare professional will obtain as reimbursement, given the patient’s health insurance coverage and agreement the Center has in place with the health service provider.
The US team is in the process of requesting a new CPT® code with the AMA for the procedure of SCENESSE® administration. By obtaining a unique CPT® -code for the SCENESSE® administration, it will facilitate and accelerate the Prior Authorization approval, medical billing, and reimbursement for the EPP Specialty Centers. Most of all, the combination of J-code and CPT® -code will introduce SCENESSE® as standard of care for US insurers adopting it on their list of reimbursable drug therapies.
As the knowledge and experience about SCENESSE® grows, the number of registered patients is still increasing while the ease of gaining access to treatment is being addressed. By now making treatment available within a radius of three and a half hours of the majority of patients, we are nearing our goal of providing sufficient options nationwide.
In parallel, we are in the process of establishing a structure in the US which will enable CLINUVEL to handle and distribute more drug products in the future. For this we follow a comprehensive plan to obtain all necessary pharmaceutical licenses in each of the key states.
A first careful assessment shows that US payments fluctuate by month and much depends on each insurer. The larger insurers seem to adhere to payment terms exceeding 120 days, while smaller ones appear to adhere to 90 days. The payments received vary per state as well, but no bad debt has been recorded thus far.
The unique J-code was assigned to SCENESSE® on 01 January 2021, and we have since seen a number of changes occurring.
Vitiligo Program
Dr Linda Teng, Director North American Operations
Since 2016, the US Food and Drug Administration (FDA) has introduced public townhall style meetings to discuss selected untreated diseases with an emphasis on patient-driven questions on new drug therapies. As we had reported before, the EPP Workshop Meeting in October 2016 was the first of these regulatory initiatives. On that day, 150 EPP families gathered to express their experience with the disease, as well as the impact it had had since childhood. Many stakeholders had travelled a great distance to attend the meeting in Maryland.
On 8 March, the FDA’s Center for Drug Evaluation and Research hosted a first-ever webinar inviting patients to discuss the impact of vitiligo (loss of pigmentation) on their lives, their experience with available treatments, and expectations for the next one. Launched as Patient-Focused Drug Development in Vitiligo, all stakeholders (but for the pharmaceutical industry) were asked to participate on the day.
In a meeting lasting four hours, a great number of patients, parents and advocacy groups had the chance to share their version of the impact of the loss of pigmentation on their lives, including their reduced chance to obtain employment and lead a socially acceptable life.
The 700 participants and FDA’s reviewers would have seen and heard from patients first-hand of the stigmatising nature of the disease. Viewers on the day would have been astonished to learn each individual’s ordeal to come to terms with the progression of the depigmentation. What was usually discovered as an initial small vitiliginous lesion had frequently become a fast involvement of large parts of the skin. Many patients of colour reported to have lost more than 80% of the body’s pigmentation and had literally turned ‘white’ and thereby had lost their identity. Depositions of emotion and despair were seen through live streaming.
The psychosocial impact, loss of self-esteem and suicidal ideation were explicitly mentioned as the many testimonies poured in from patients and parents.
The majority of the on-screen invitees were patients of colour. While in darkly pigmented colour the loss of pigmentation is most apparent, the racial abuse suffered by patients has been an additional factor in most ‘vitiligo journeys’.
Parents spoke about their children being bullied due to the disfiguring loss of facial pigmentation, and other patients commented how they had not obtained the desired professional position having had a face-to-face interview, since it was subsequently discovered that the employer feared that the hiring of a vitiliginous employee would scare off the clientele and be bad for business.
For months our scientific and clinical teams had anticipated this meeting, since they were eager to know whether new facts would contribute to our next FDA discussion – later this year – on the final protocol design for a Phase IIb/III trial.
On the day, the FDA posed predetermined questions during various patient-led panels, giving a platform to provide clarity on specific subjects. We share a number of poignant answers to FDA’s questions which came out of these stakeholder-driven sessions:
1. The US vitiligo spokesman and news anchor Lee Thomas spoke on behalf of patients stating that the meeting was seen as a first recognition by the FDA of vitiligo not being a cosmetic disease but a stigmatising one which needs to be taken seriously by society and regulators, and which had previously not deserved national attention.
2. Most patients of colour are self-conscious and experience abuse, discrimination, and avoidance by their environment.
3. The FDA and drug companies should not only pay attention to developing therapies but also to the psychosocial impact of vitiligo.
4. A frequent concern and problem for vitiligo patients is the tendency to burn under the sun, while mineral sunscreens do not offer protection.
5. A common and unreported problem for patients is the frequent itchiness of the vitiligo lesions.
6. Facial loss of pigmentation is most impactful, since the face is the feature which is central to daily communication.
7. Drugs that potentially suppress the immune system systemically or locally are regarded as a risk since one does not know the possible long-term effects.
8. Cure would be an aim, but any therapy which lessens the burden of narrowband UVB (NB-UVB) would be very helpful; since NB-UVB requires patients to attend the dermatology clinic thrice per week for 12 to 18 months duration, they are often unable to attend and remain compliant, which in turn leads to lack of treatment effect.
9. A minimum required goal for a new therapy would be to achieve repigmentation of more than 50% of the vitiligo lesions on the face.
10. A desperate cry to FDA and drug companies to develop an effective therapy for adults and children.
The FDA’s open docket is closing on 10 May, until which time all patients and the public have a chance to share new relevant facts on vitiligo.
CLINUVEL’s team will be meeting the FDA one more time to agree the final protocol design for vitiligo whereby all aspects summarised during the workshop of the 8 March will be incorporated.
My impression of the day was that the FDA is certainly now taking vitiligo seriously, and although no approved therapy has ever been made available for the disease, I sincerely believe the agency has openly expressed its commitment to support drug development for these patients.
I also took away from the meeting that immune-suppressive therapies in development for vitiligo are a real concern to patients, and the medical community will carefully consider these before administering to a patient. If, and when, immune-modulators receive regulatory approval for vitiligo, there will be wide-spread caution and anxiety about the long-term effects.
Importantly, the workshop highlighted again the lifelong risk of sunburn in vitiligo patients, and the need to photoprotect them.
Surprisingly, the symptoms of persistent itching in vitiligo have not been well described thus far and have not been prominent in drug development.
Although, the FDA has not approved NB-UVB as standard therapy in generalized vitiligo (the most common clinical presentation of the disease), it is currently the preferred treatment for patients of colour. Some patients have relied on handheld UV-emitting devices for home use to avoid the time-consuming visits to medical offices, but frequent burning accidents are reported to have occurred.
We refer to our Vitiligo Scientific Communiqués to review our work and scientific progress in generalized vitiligo. The use of afamelanotide in combination with NB-UVB was first published in JAMA Dermatology and later in the Journal of the American Academy of Dermatology. On 29 October 2020 in our Strategic Update I, we provided a summary of vitiligo.
The overall treatment of vitiligo patients calls for psychosocial evaluation and possible counselling to ease mental stress during treatment, described as a possible co-factor in the development of vitiligo.
Our teams came away with excitement about the FDA’s initiative to finally seek patients’ input to learn more about vitiligo and to support drug development. We will report once the final Type C meeting with the FDA has been held and agreement on a protocol reached.
Current Vitiligo Treatments (USA) Off-label (unapproved for vitiligo):
– Corticosteroids (topical)
– Calcineurin inhibitors (topical)<br
– vitamin D Derivatives
– PUVA-psoralen</br
– Narrow-band UVB
– Synthetic prostaglandins F2-alpha analogue
– Janus kinase 1 and 2 inhibitor
– Depigmenting Agent
– Prostaglandins (topical gel)

“I have faced many challenges living with this condition in a society that is focused on physical appearance. These include uncomfortable stares, finger-pointing, people refusing to touch my skin for fear that it is contagious and hurtful name-calling”.
– Dhyana, living with vitiligo for 11 years.
EU Distribution
Lachlan Hay, Director of Global Operations
The curfews and restrictions implemented across Europe continued to impact the day-to-day lives of the EPP patient community as well as the physicians with whom we work. Our staff works around their availability and much of the post-authorisation monitoring currently occurs at distance. In spite of the challenges, it is encouraging to report that most of the active European EPP Expert Centres have started treating patients for the calendar year, with new centres set to start.
Across Europe, the team has continued to follow the post-marketing requirements imposed by the EMA in 2014 to monitor the prescription of SCENESSE® to EPP patients. Here the focus is on the safety profile of the product over the long-term as well as management of specific identified and potential risks under an agreed Risk Management Plan. It is testament to the long-standing relationships with the academic and clinical experts providing care to their patients, as well as the commitment of the CUV team, that the majority of EPP patients choose to enrol in the post-authorisation safety study (PASS) while agreeing to their data being pseudonymously uploaded to the European EPP Disease Registry. This provides our team with near-real-time feedback on the use of the drug as well as the ability to provide data-rich reports to the EMA on an annual basis.
The PASS Annual Report is a major body of work undertaken by our Clinical Operations team. Like any statistical report, it relies not only on the quality and accuracy of the data and the analysis, but on a fundamental understanding of what these results show. Put differently, without expert knowledge of how EPP patients are being treated in the clinic, the statistics are of limited use. Being able to rely on a consistent team and in-house knowledge year-on-year provides great value.
The most recent Report – the fifth, completed in December and reporting data up to 22 June 2020 – gives insight to the ongoing use of SCENESSE® across Europe. Paramount to our analyses is that the safety profile of the product has proven consistent both with previous years and within the confounds of the approved product label. Three in five patients report some adverse reaction to the treatment (nausea and headaches are most common), but the majority of these reactions are mild and temporary (resolving within 24-48 hours post-treatment).
Year-on-year treatment, the number of patients continuing to receive SCENESSE® remains high: our latest analyses show a 94% adherence to annual treatment. Measures of patients’ quality of life, when compared to baseline, show an improvement in response to SCENESSE®, as well as a year-on-year improvement in the spring and summer months, when
patients are at their highest risk of phototoxic reactions.
The Fifth Annual Report filed to the EMA reflects the clinical evidence and peer-reviewed journal articles being generated from European EPP Expert Centres, which account for an overall improvement in patients’ lives and the ability to lead a “normal” existence previously thought impossible. While this gives comfort that we provide significant and real benefit to patients, our teams remain committed to ensure we can provide lifelong care to EPP patients.
The challenges of the pandemic and evolving regulatory landscape have extended across the business, with changes required to facilitate compliance in an evolving and unpredictable environment. Here, foresight from our team, engagement with regulatory authorities, and work with our distribution partners has facilitated treatment availability.
One illustration is the approach taken to batch release of finished pharmaceutical products. The processes of batch release have recently received some high-profile media mentions in the context of COVID-19 vaccines, but few have paid attention to the role batch release plays in the supply of the specialised pharmaceuticals. At its core, batch release requires an independent “Qualified Person” (QP) to analyse and certify documentation, confirming that a drug product meets the quality and specifications necessary to make that batch available for patient use. Safety is the central concern – without a consistent, quality product, a company is unable to ensure the safety profile of a pharmaceutical product. As one would expect, this task is highly regulated, and conducted according to strict quality management protocols, dictating the physical location at which release takes place – a certified Good Manufacturing Practices (GMP) site. This approach ensures the release process is consistent and controlled. Over the past year, however, the ability to visit and audit GMP sites has been restricted (if not totally impossible) in many countries, forcing a re-think of how and where batch release takes place and the overall requirements of quality systems. While it may seem simple, enabling the expansion of a quality system to facilitate remote batch release is a decisive shift in regulatory thinking, but one that has allowed CLINUVEL to continue drug supply. One foresees that this practical change – and others brought about by the pandemic – will continue long after restrictions are lifted. New environments force us to rethink and redesign our systems.
As to pricing of SCENESSE® in the European Union, we aimed to lead by example and respected the national agreements we have made the past years. These pertain to:
I. net uniform pricing
II. number of prescribing EPP Expert Centres
III. projected volume, number of injections
IV. number of patients
V. off-label use
VI. trained & accredited centres, and
VII. post-marketing surveillance.
In a world where wide-spread scepticism dominates during the interactions between insurance groups and pharmaceutical companies, we designed a unique plan to demonstrate to the payors a transparent and uniform approach.
In many of these discussions, negotiations and – in a number of instances – court cases and appeal panels, we laid down CLINUVEL’s mode of operating and how we would maintain a net uniform price across all member states and the Eurozone. Our teams never deviated from this principle, and we refrained from increasing the price over the past four years.
As a norm, the overwhelming majority of drugs do incur price hikes and adjustments for index-based consumption. However, CLINUVEL did not correct for CPI despite the rising costs of raw materials, manufacturing, labelling, packaging, distribution and transport. This steadfast belief in a strong principle would even invite critique from funds and institutional shareholders, however we maintain the belief that transparency in this domain goes a long way and commands, at the very end, the respect of the same payors honouring contracts with the pharmaceutical actors, such as CLINUVEL.
Today, in the fifth year of commercial distribution of SCENESSE®, we can speak to the same insurers per country and provide quarterly reports detailing how we have honoured the agreements made from 2014 to 2021. CLINUVEL has not exceeded the number of centres, number of injections and patients treated in any of the European countries, which is congruent with the national submissions we have made from 2014 onwards.
Importantly, the general anxiety was that CLINUVEL would “flog” the drug to markets and distribute off-label. Here again, we adhered to our stated principles and agreements, and not a single SCENESSE® implant has been supplied for off-label use. I do, however, need to caveat this statement. Our teams provided one off-label implant for a congenital erythropoietic porphyria (CEP) patient who was terminally ill; we did this on a compassionate basis to try to provide a quality of life in the final months of the patient’s life.
The question is often posed whether CLINUVEL therefore has maximised the commercial potential of the afamelanotide drug. Paradoxically, we can wholeheartedly answer that by operating in this fashion we actually are maximising the opportunity. By restricting the wide-spread and negating the off-label use, we have gained the trust of many payors and insurance groups and this provides a fertile discussion for years to come, perhaps even serving as a benchmark to other companies.
As of September 2021, CLINUVEL will adjust the SCENESSE® price to reflect the annual price increases at more than 20% we have borne over the past four years for hikes of all raw materials and manufacturing processes. Our price increase will be discussed with payors and insurers and amounts to approximately 13.1%. Three months before the price increase becomes effective, we will make this known to the respective insurance groups.
It is testament to the long-standing relationships with the academic and clinical experts providing care to their patients, as well as the commitment of the CUV team, that the majority of EPP patients choose to enrol in the post-authorisation safety study (PASS)
The processes of batch release have recently received some high-profile media mentions in the context of COVID-19 vaccines, but few have paid attention to the role batch release plays in the supply of the specialised pharmaceuticals
In many of these discussions, negotiations and – in a number instances – court cases and appeal panels, we laid down CLINUVEL’s mode of operating and how we would maintain a net uniform price across all member states and the Eurozone.
Expansion CLINUVEL Group
In 2020, we revealed the second afamelanotide formulation, PRÉNUMBRA®, as part of the molecule’s life-cycle management. We have long planned to use the molecule and other melanocortins in life-threatening and severe disorders.
The commercial success of PRÉNUMBRA® hinges on a number of aspects. First, the safety profile of the products used in various disorders needs to resemble the known safety data of SCENESSE® as we currently have from adult patients with EPP or photodermatoses.
Second, and of equal importance, for the product to be used commercially, our emphasis is given to the development and validation of analytical methodologies. In simpler terms, the way we measure the chemical and pharmacological properties and biological behaviour of these two products within the human blood circulation requires sensitive and specific methodologies (assays) developed by our analytical teams in the Singapore laboratories. These aspects and commercial avenue of PRÉNUMBRA® will get attention in Strategic Update II.
Despite the opportunity globalisation offers through connectivity, I paradoxically see strong geopolitical trends towards a retreat to national self-sufficiency and locoregional focus. I have gradually viewed sovereignty of relevance to dependent and expanding organisations such as CLINUVEL. In witnessing national economies retreating, surplus countries suffering from waning exports and the impact of dependence being played out, choices were to be made as to CLINUVEL’s future and its risks.
The Board of Directors, consultants, legal teams and managers-at-hand had discussed the option of manufacturing for many years. For this we required sustainability of the business, belief in future markets and analysis of current treatment options. As we added OTC products – non-prescriptive products for wider public – to our portfolio, we affirmed our need to become independent and bring the fourth division in-house.
Naturally, debates can be held as whether operating manufacturing facilities is prudent in current economic conditions. In CLINUVEL’s case, however, the benefits clearly outweigh the negatives. It requires focus, cost-management and operational expertise. In focussing on sustained-release products, but also transdermal formulations, the Group will be in the position to manufacture as a Contract Development & Manufacturing Organisation (CDMO) for other research groups and companies. Our Chairman has most recently written about the addition of this fourth division.
In CLINUVEL’s case there is a synergy between our team at VALLAURIX (Singapore Laboratories) and manufacturing, while we foresee that personnel will interchange. The manufacturing facilities will comprise analytical laboratories on-site whereby method transfers will occur within the Group.
It is a riveting part of our expansion, and one which has required much thought, attention, planning and new skills. The addition of newly recruited managers facilitated discussions on design, engineering, equipment, processes and products.
In the October 2020 Strategic Update I the Company announced the creation of a new Communications, Branding and Marketing (CBM) Division, which is being established largely in the UK. This approach is in line with CLINUVEL’s modus to build and retain talent under one roof over the long-term, rather than relying on external expertise. The objective is to create an agency-style team that services CLINUVEL, enabling the Company to engage with new audiences in a modern and creative manner. This has necessitated adding new competencies to the Group, investing in talent and expertise, software, systems and equipment. Currently, we report that the recruitment of the CBM team is 80% complete, with four more vacancies to be filled to be able to execute all media and communications functions digitally.
The CBM Division has been set clear targets to grow the online presence of new brands, to address audiences with the projects and products we plan to launch. As a result, investors will see how CLINUVEL will gradually shift in presenting itself over the coming months.
The Board of Directors, consultants, legal teams and managers-at-hand had discussed the option of manufacturing for many years.
In the October 2020 Strategic Update I the Company announced the creation of a new Communications, Branding and Marketing (CBM) Division, which is being established largely in the UK.
Investor Relations
Malcolm Bull, Head of Investor Relations
CLINUVEL has continued to deliver positive news on its progress in the first quarter of 2021.
During this period the Company released two key financial updates. The first, in January, was the Cash Flow Report for the December quarter 2020 and the second, in February, was the Half Year Report for the period ending 31 December 2020. Cash receipts drove a positive net cash outcome in the December quarter 2020, compared to negative net cash receipts in the same period in 2019 and 2018. For the 2020 calendar year, cash receipts achieved a record level of A$33.053 million. For the half year ended 31 December 2020, CLINUVEL reported the tenth consecutive half year profit and a record profit for a December half year.
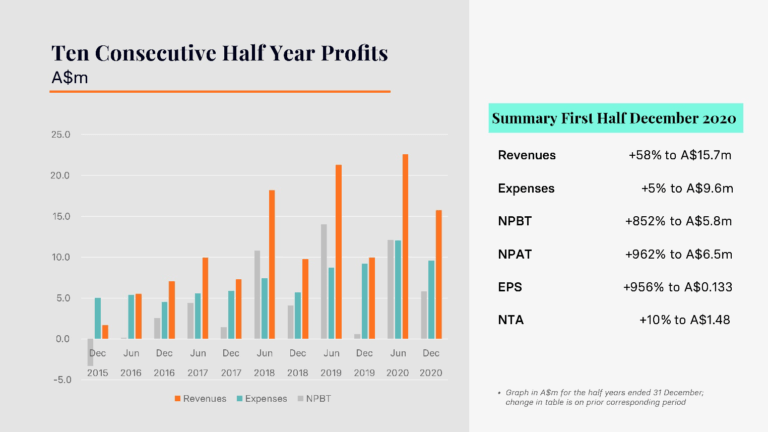
Revenues rose 58% and expenses were well managed to a 5% increase, producing a net profit before tax of $5.811 million for the first half of the 2021 financial year.
The cash and profit results reflect the normalisation of treatment activity in Europe after initial restrictions on treatments related to the COVID-19 pandemic in the March – May period of 2020, and the first contributions from the distribution of SCENESSE® in the USA.
Operational update – webinar.
The Company has diversified its communications this year by commencing an operational update webinar series, to provide an alternative form of communication on the progress of the business to complement formal Company announcements. In the first webinar in February, CLINUVEL executives covered the half year results to 31 December 2020, and provided an update on the distribution of SCENESSE® and the expansion of the research and development program. The webinar was well received, with positive comments on the format of the discussion, as well as the effective use of graphics to complement the commentary. We find this feedback encouraging to continue this initiative.
We will again call for questions from shareholders ahead of the recording of the next webinar to help guide the conversation.
Other announcements
In February we announced the grant of market access of SCENESSE® in Israel, which is the first approval of national reimbursement of the cost of treatment of SCENESSE® in the Middle East. Last month we announced the expansion of the DNA Repair Program to evaluate SCENESSE® in patients with xeroderma pigmentosum variant, XP-V. The expansion of the program is based on ongoing discussions with clinical and academic experts, who support the use of the drug in both the XP-C and XP-V complementation groups. There is a strong scientific rationale to use the drug in XP-C and XP-V, and there is sufficient support to make this logical expansion. The last week, we unveiled our expansion with a fourth division, manufacturing.
All the Company’s announcements are available on the CLINUVEL website.
The second Strategic Update is to be released in the coming weeks and will build on the October 2020 Strategic Update.
Participation in key events
In 2020, we participated in eight key conferences and presented at six, mainly virtually, as has become the temporary norm.
Our objective is to reach new potential investors by telling the CLINUVEL story and conveying the compelling reasons to consider us as an investment, and to inform existing investors of the progress of the Company.
CLINUVEL has participated in three prominent international conferences so far this year. The first was the JP Morgan Healthcare Conference in January. We then presented at the HC Wainwright Bioconnect Conference in January and the Daiwa Investment Conference, Tokyo in March. All of these were virtual conferences and, whilst this does limit the extent of relationship building typically achieved through face-to-face meetings, we are pleased to be reaching new investors and having one-on-one meetings with those most interested in CLINUVEL.
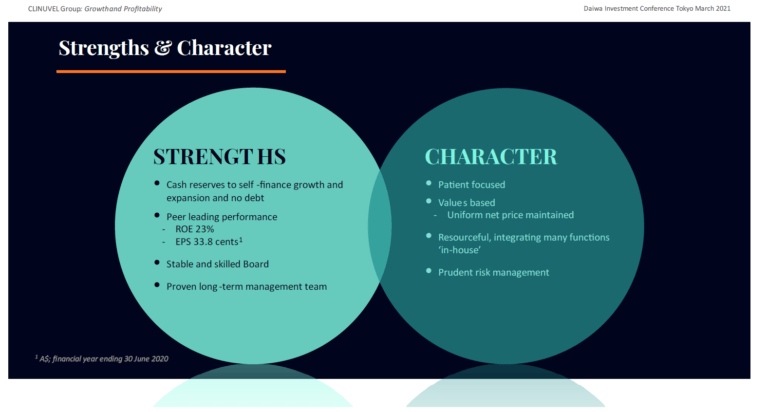
The presentations covered the evolution of CLINUVEL through years of drug development to the achievement of positive cash and profit generation and a strong financial position from commercial operations. The role of the Company’s tenacious character in these achievements, and the expansion of the research and development program, with the planned deliverables for 2021, were also covered. The strengths and character of CLINUVEL presented at the conferences is shown below to illustrate how we present the Company to external stakeholders.
The presentation and our story have been well received with a follow-up expressed by institutional investors. This requires our time and further discussions.
The calendar of planned events for 2021 is provided below for your information, noting a number are yet to be confirmed.
| Month | Planned Events |
|---|---|
| January | JP Morgan Healthcare Conference, San Francisco |
| HC Wainwright Bioconnect Investment Conference | |
| March | Daiwa Investment Conference, Tokyo |
| May | UBS Global Healthcare Conference (tbc) |
| European Society for Paediatric Dermatology, 20th Annual Meeting (tbc) | |
| June | Jefferies Global Healthcare Conference |
| Goldman Sachs Global Healthcare Conference (tbc) | |
| American Society for Photobiology 2021 Symposium (tbc) | |
| September | Goldman Sachs European Medtech & Healthcare Conference (tbc) |
| HC Wainwright Global Investment Conference (tbc) | |
| October | Morgans 5th Annual Value in the Vines Investor Conference (tbc) |
| CitiÕs 12th Annual Australia & New Zealand Investment Conference (tbc) | |
| November | Jefferies London Healthcare Conference (tbc) |
| XII International Congress of Dermatology |
The webinar was well received, with positive comments on the format of the discussion, as well as the effective use of graphics to complement the commentary.
Summary
COVID keeps occupying great parts of the world, while Australia and New Zealand appear to have mastered the spread of the virus effectively.
Our Singaporean laboratories (VALLAURIX) are back working full time, operating one shift since the circuit breaker has been lifted, and there is no longer a national distinction between essential and non-essential services. Our staff have welcomed new members during the COVID restrictions, and integration is seamless owing to our efficient processes.
In Europe, England, France, Germany, Italy and the Netherlands are imposing curfews and restrictions out of office hours, and much uncertainty rules whether economic life will resume swiftly. I believe that 70% of the population will need to be vaccinated before EU governments will contemplate lifting restrictions.
Recently, in a discussion with an Australian fund manager the subject was broached as to the most important factor had been in CLINUVEL’s successful journey to date. Without time to prepare the meeting, I answered him by providing a favourite parable uttered by a young spirited lawyer and great American, Abraham Lincoln: ‘always bear in mind that your own resolution to succeed is more important than any other one thing’.
The fleeting nature of success in pharmaceuticals compels me to look away from our favourable status, but I take comfort in knowing that our team has shown resilience and ingenuity to overcome obstacles where most others would have given up; while I am averse of providing guidance in public companies, looking at consistent human behaviour and execution for the past 15 years is perhaps the closest one comes to prophecy. We rely on a great assembly of professionals willing to devote their time and energy to great causes.
With CLINUVEL we are on an odyssey, have stared into the abyss, and gazed at peaks. I am sure quite a number more challenges lay ahead, but based on the execution of my senior managers, a consensual Board, I am convinced that we will achieve all our objectives in time. I lead a life set by the principle that once the human mind is set to specific tasks, a concentrated effort will provide results, such is the power of individuals sharing these genuine beliefs. We have that at CUV.
We will meet again in the next weeks for the Strategic Update II, when we provide further direction to CLINUVEL’s expansion and reasons underlying these decisions.
Philippe Wolgen
I take comfort in knowing that our team has shown resilience and ingenuity to overcome obstacles where most others would have given up
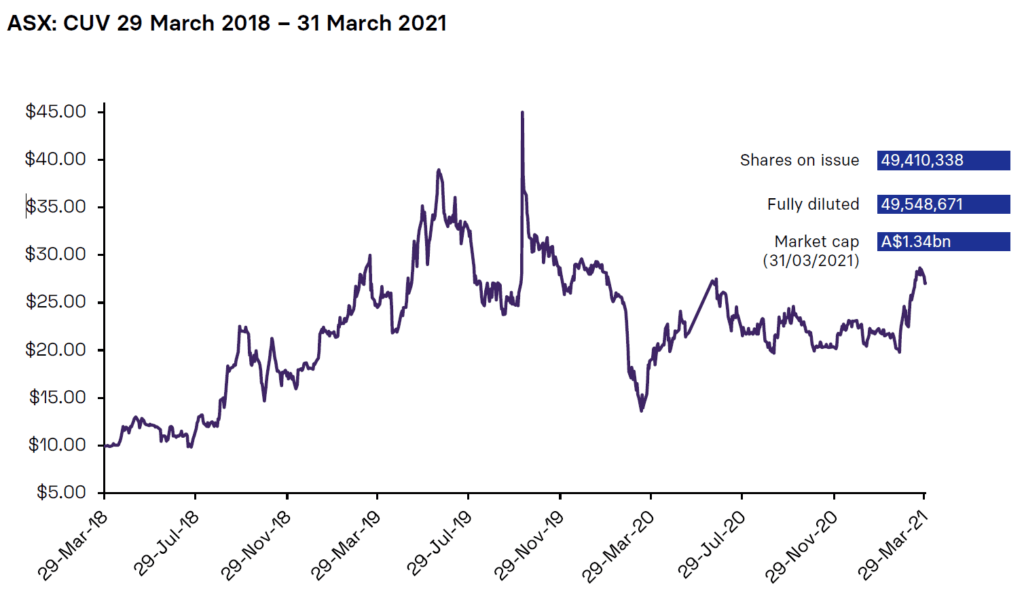
Share Price
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD.
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance, or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products, the COVID-19 pandemic affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical products; competition for our products, especially SCENESSE® (afamelanotide 16mg); our ability to achieve expected safety and efficacy results through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, China and Japan of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE® which may lead to it being unable to supply its commercial markets and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; any failure to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2020 Annual Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on the forecasts and estimates is available on request. Past performance is not an indicator of future performance.