CLINUVEL Communiqué IV
| Melbourne, Australia, 23 August 2022 | ASX: XETRA-DAX: Level 1 ADR: |
CUV UR9 CLVLY |
Dear Shareholders, Friends
Introduction
(Mr Malcolm Bull, Head of Australian Operations & Investor Relations)
CLINUVEL’s key activities
Since the last News Communiqué on 23 June, CLINUVEL announced the development of the liquid formulation of afamelanotide, PRÉNUMBRA® Instant, for first use in the next clinical study (CUV803) in arterial ischaemic stroke (AIS).
We also advanced clinical studies in DNA Repair, focussed on xeroderma pigmentosum (XP) patients and disease-free subjects, and worked towards the first study of SCENESSE® (afamelanotide 16mg) as a monotherapy in vitiligo.
In the background, our Communications, Branding & Marketing team has been preparing three online campaigns, reaching specialised audiences at ‘Highest Risk’ of photodamage and skin cancers. In our prospective Strategic Updates, more colour on these campaigns will be provided. CLINUVEL’s approach to the specialised populations differs from the commonly practised online consumer campaigns. In the first instance, the feedback received from the targeted communities is very positive for raising the awareness of the high risk of photodamage.
Reflecting the growth of SCENESSE® distribution, cash receipts grew strongly during the final quarter of FY2022. The positive net cash generated throughout the June quarter boosted cash reserves, further adding to the Company’s ability to self-finance its key initiatives and manage the impacts of the economic cycle. The Company’s cash flows are discussed in more detail in this Communiqué.
Ongoing volatility into the new financial year
As we have entered a new financial year continuing to operate in a volatile environment, there is an even higher need to remain focussed on key objectives. We strive to fulfill our mission to establish a diversified company with multiple technologies, services and income streams. As an international company addressing patient needs in three regions, Europe, the USA, and Israel, we have no choice than to keep a close eye on world economic and political developments and possible impact on our operations.
The current focus among economists is on interest rates, and the extent of increases to be expected to control inflation. The trade-offs seem to be the likelihood of recession, and unemployment. Some are predicting a recession looming in the US, and other developed economies, while opinions vary.
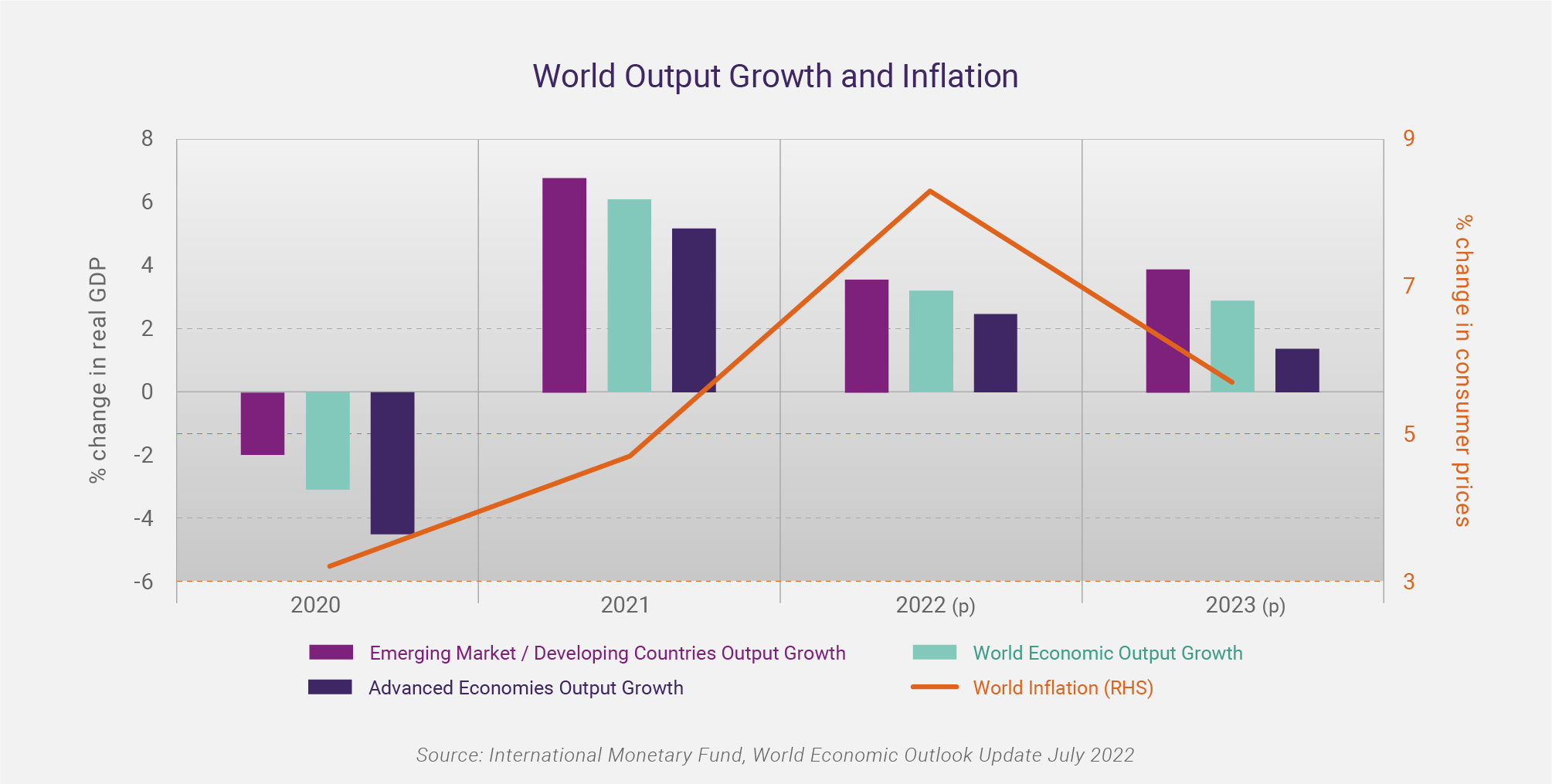
The International Monetary Fund (IMF), in its World Economic Outlook Update July 2022, has forecast global economic growth to slow from 6.1% in 2021 to 3.2% in 2022, and 2.9% in 2023, based on a “gloomy and more uncertain” outlook. In the advanced economies, the forecast is for an easing in economic growth from 5.2% in 2021 to 2.5% in 2022, and 1.4% in 2023. Whilst the outlook is still for positive economic growth relative to the COVID-19 induced recession of 2020, the IMF states “the risks to the outlook are overwhelmingly tilted to the downside.”
There is at the same time the debate how global markets will react to uncertainty about monetary policies.
Early signs of recovery in the biotech sector?
It is widely recognised that publicly listed biotechs in major markets have been extensively sold down relative to other sectors. It is only in the last month or so that we see signs emerging that the sell down of the biotech sector may have abated, and values starting to recover.
As an indicator of distress in the sector, one only needs to look at the number of life science companies worldwide whose enterprise value is lower than their cash reserves. Figures issued by Torreya Capital show the number of distressed companies increased from 21 on 9 January 2021, to a peak of 220 on 11 May 2022. This eased to 203 at the beginning of July 2022 and has since improved to 157 on 12 August 2022.
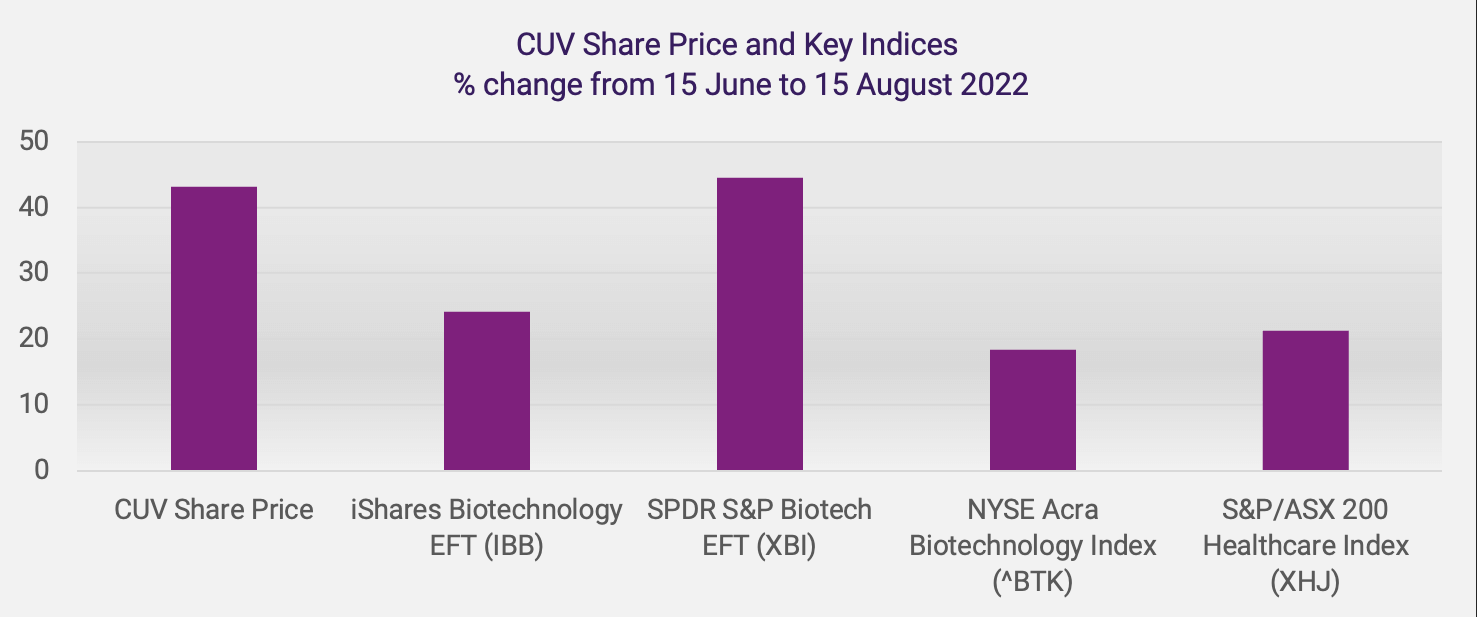
H.C. Wainwright notes that key US life sciences indices “strengthened and materially outperformed the major equity indices” in the period from 16 June to 15 July 2022. They report rises in the SPDR S&P Biotech EFT (XBI) of 28.8%, the iShares NASDAQ Biotechnology EFT (IBB) of 16.5% and the NYSE Arca Biotechnology Index (^BTK) of 13.4%, compared to rises in the NASDAQ of 7.6%, the S&P 500 of 5.4% and the Dow Jones Industrial Average (DJIA) of 4.5%, during this 30-day period.
CLINUVEL saw a recent low closing share price of A$13.74 on 15 June and witnessed a recovery to A$19.66 on 15 August, an increase of A$5.92 or 43.1%. The change in CUV’s share price compares favourably to the change in key indices in the US and Australia over the same period, as shown below.
It is only in the last month or so that we can perhaps see signs emerging that the sell down of the biotech sector may have abated…
CLINUVEL’s position in current markets
The strategic path we have followed has led to our current position ranking among a relatively small group of revenue generating and profitable biopharmaceutical companies in the sector. A strategic consideration was to establish en entity which would withstand economic cycles, and which would appeal to those seeking robust biopharmaceutical companies. Knowing that many biopharmaceutical innovators do not reach profitability, we intended to navigate some of the pitfalls. From a position of strength, we move forward to self-finance the development of new products for a wider range of indications, all characterised by ‘unmet need’. CLINUVEL’s positive fundamentals encompass:
- a profitable business, generating incremental net cash with a highly liquid balance sheet, and no debt;
- significant potential to leverage its expertise in melanocortins;
- the translation of technology and knowhow to a non-pharmaceutical product;
- an integrated business model in which many functions are undertaken ‘in-house’; and
- a stable management team, highly committed.
In this Communiqué
Ahead of the release of CLINUVEL’s financial results for the 2022 financial year, we cover in this fourth communiqué of the year a range of topics:
- afamelanotide for erythropoietic protoporphyria (EPP);
- the DNA Repair Program;
- Cash receipts for the June quarter 2022;
- the uses of the accumulated cash balances of the Company; and
- recent and planned company announcements and events.
Afamelanotide for EPP: addressing the challenges of a unique, complex disorder
(Mr Lachlan Hay, Director of Global Operations)
Of course, expert physician and regulatory support, and agreements with global payors, have been the cornerstones of successful commercialisation of SCENESSE®. However, more factors play a role to ensure continuous commercial viability. Today, six years after market entry, SCENESSE® is the standard of care for adult EPP patients in the European Union, the USA, Switzerland and Israel, with the team focusing on facilitating access in Australia. Year on year we continue to expand access to the treatment, with the goal of enabling access for all EPP patients, requesting treatment.
For new readers, EPP is a rare disorder of haem biosynthesis, affecting an estimated 5,000-10,000 individuals worldwide. Due to an enzymatic deficiency, EPP patients accumulate a phototoxic compound – protoporphyrin IX or PPIX – in blood and liver. When exposed to certain wavelengths of light, predominantly visible light peaking at 408nm, PPIX reacts, creating reactive oxygen species which damage surrounding tissue. Clinically, EPP patients will experience debilitating phototoxic reactions when they expose to light sources – even briefly – within the visible spectrum. These anaphylactoid reactions are described as a deep burning sensation, accompanied by erythema (redness) and oedema (swelling). The reactions can last days to weeks and are unresponsive to analgesics, leaving patients largely unable to function normally.
CLINUVEL’s approach to treating EPP patients has always been founded on assessing safety first, commencing the program with a strong dossier of data supporting the use of a melanocortin drug as an interventional treatment.
Safety first
As reported many times, CLINUVEL’s approach to treating EPP patients has always been founded on assessing safety first, commencing the program with a strong dossier of data supporting the use of a melanocortin drug as an interventional treatment. The therapeutic approach in EPP is based on a deliberate targeting of a receptor (melanocortin 1 receptor or MC1R) with an analogue of our body’s own hormone (α-melanocyte stimulating hormone or α-MSH) to allow the activation of a natural signalling pathway. In the sense of biomimicry, activating the body’s own cellular responses to elicit a response, pharmacology provides for more predictable biological responses, particularly when there are minimal, controlled, changes to the natural hormone.
The other advantage of this approach is that the natural pathway has been well studied over decades, as opposed to approaches which intervene midway through a pathway, with unknown peripheral effects. This shouldn’t lead to complacency but does allow for a degree of comfort for the long-term repetitive dosing required in a lifelong disorder. Meanwhile, based on the pharmacology of afamelanotide and the deliberate choice of the route of administration, a minimal dosage strength is required for its clinical efficacy. A single 16mg dose, administered every two months in EPP, is sufficient to elicit the desired pharmacodynamic response and photoprotect patients. This approach to dose minimisation reduces the risk of unwanted adverse reactions, while giving patients limited overall drug exposure.
Our program continues to generate data from the post-authorisation use of SCENESSE®, enabling our teams to learn much about the daily use of the drug. Some aspects of current use and demand are very different to what could have been predicted during clinical trials, one of the reasons why our teams follow up patients longer-term, and why frequent contact remains essential with prescribers.
Back to the long-term safety argument, in our plans of translating the use of melanocortins, the details on safety data received and analysed can make all the differences between filing for – and securing – patents, entering a clinical program and choosing appropriate objectives (endpoints) for studies and regulatory review.
We have followed several national cohorts of patients long-term, with many well into their fourth or fifth year of treatment. The longest treated patients are into their sixteenth year of therapy, receiving 75 or more SCENESSE® implants. In total, over 10,000 doses have been administered. We have also established the world’s largest EPP disease registry, allowing longitudinal care for patients, which we analyse annually for regulatory submissions. We have also seen some data in special cohorts, with a number of patients over 70 now treated, as well as patients taking breaks from treatment to start families and returning to treatment thereafter. To date, we have seen a remarkable consistency in safety data generated among all cohorts.
The longest treated patients are into their sixteenth year of therapy, receiving 75 or more SCENESSE® implants.
Three objectives
With a commercial foundation and safety record established in afamelanotide for adult EPP patients, there are three immediate objectives. First, accumulating safety data to enable the translational use of afamelanotide in two life threatening disorders, one of which is XP.
Second, validating the first findings – as we reported in News Communiqué I earlier this year – that afamelanotide is actually providing longer-term liver tissue protection in EPP patients, up to 20% of whom incur some form of liver damage.1
Third, to allow the drug to be used in paediatric patients, below 18 years of age.
CLINUVEL is actively working towards expanding treatment access for paediatric EPP patients, for whom no treatment exists. Arriving at this point has required parallel progress across several key metrics – including clinical, regulatory, and commercial – to maximise our chances of acceptance of paediatric EPP treatment with afamelanotide.
In parallel, we continue to see independent literature reports confirming the ongoing clinical benefit of afamelanotide treatment. Our annual report to the US and EU regulatory authorities – while focused on safety – provide analyses of drug effectiveness using disease-specific tools. One of the scientific tools is the EPP-QoL, a relatively new way of capturing impact of disease on patients’ lives. In using this tool, peer-reviewed publications report improvements in patient quality of life following afamelanotide treatment. All information points to one direction. A new finding indicates that EPP patients receiving treatment experience a normalisation in circadian rhythms, further evidence of the changes to patients’ conditioned behaviour following treatment.2
Looking ahead
Briefly, there is much more we are seeking to achieve for the EPP community. Later this year our team will sponsor and attend the International Congress of Porphyrins and Porphyrias in Sofia and provide support to the American Porphyria Foundation to attend this event. Such conferences allow our team to maintain contact with the latest research and developments in the field.
Commercial and regulatory work is also continuing. In addition to widening access to all adult patients, who request therapy, the Company is now preparing paediatric access, a new milestone for the first EPP treatment. We expect to share more on this program later this year.
The DNA Repair Program
(Dr Pilar Bilbao, Head of Clinical Operations)
For our newly joined readership, I take a moment to summarise the rationale and aims of CLINUVEL’s DNA Repair Program.
Our teams broke new ground in providing total photoprotection in EPP, to eventually fulfil a clinical objective of providing assisted DNA-damage repair to XP patients, who – due to a genetic disorder – are globally viewed as having the highest risk of photodamage and skin cancer. The EPP and XP programs together, give us the knowledge, data and safety to translate our technology and experience to other specialised groups at Highest Risk of photolesions and skin cancer.
In translating science, data and experience give one genuine authority to speak about relevant topics, such as photoprotection. This is part of our mission, doing good to benefit a few first and many of us later; the need for adequate photoprotection is huge.
Overview of the program
CLINUVEL’s clinical program to assess the safety and efficacy of afamelanotide for the treatment of XP patients is expected to comprise five clinical studies, three of which have commenced. The studies are summarised below:
| Study | Patients | n = | Commenced | Objectives | First results |
|---|---|---|---|---|---|
| CUV156 | XP-C | 6 | October 2021 | Safety and reduction in oxidative damage | 2022 |
| CUV151 | Disease-free subjects | 10 | December 2021 | Reduction in oxidative damage | 2022 |
| CUV152 | XP-V and XP-C | 6 | February 2022 | Safety and reduction in oxidative damage | 2022 |
| CUV153 | XP-V and XP-C | 6 | Planned | Safety, assist DNA repair, quality of life | To be advised |
| CUV154 | XP-V and XP-C | 20 | Planned | Safety, assist DNA repair, quality of life | To be advised |
The studies already commenced, involve the taking of skin samples (biopsies) of exposed skin areas for laboratory analyses of DNA damage before and after drug administration. CLINUVEL has collaborated with expert physicians to develop new global assessment tools and patient reported outcomes for use in the studies. In addition to confirmation of the safety of SCENESSE®, the objective is to see a reduction in oxidative damage in the skin after drug administration. First read outs of these studies are expected to issue later in 2022, subject to full recruitment and completion of treatment. Pending the results of these initial studies, two further studies, CUV153 and CUV154, are planned.
The five studies aim to involve 38 XP patients and 10 disease-free subjects. The results of the studies shall be assessed to determine the next phase of the program. Of course, there is regulatory consultation to gauge agencies’ final views of the safety and efficacy of the treatment, with a view to fulfilling the requirements to gain regulatory approval for afamelanotide.
Assisted DNA repair would reduce the overall disease burden and could dramatically improve quality of life and life expectancy in XP.
The relevance of assisted DNA-repair in XP is – among other considerations – to provide further basis for translating science and knowhow to dermatocosmetic products for the benefit of wider audiences. We define these as specialised populations at ‘Highest Risk’, – refer to the Light Skin Science website.
I am excited about this journey, and how this all will come together. My team fully understands the impact of the EPP program on XP, and – in turn – its significance on larger populations.
Of course, there is regulatory consultation to gauge agencies’ final views of the safety and efficacy of the treatment, with a view to fulfilling the requirements to gain regulatory approval for afamelanotide.
Cash Receipts – June Quarter 2022
(Mr Malcolm Bull, Head of Australian Operations and Investor Relations)
The quarterly result
The table below sets out the cashflow highlights for the June quarter 2022.
| Item | Q4 FY2022¹ | |
|---|---|---|
| Cash receipts2 | $24,053,000 | |
| Operating cash expenditures | $5,624,000 | |
| Net operating cash flow3 | +$18,478,000 | |
| Cash reserves4 | +19.3% | |
| Debt-free | ||
| 1. Period 01 April to 30 June 2022. All dollar figures in this release are rounded and reported in Australian dollars.
2. Excludes interest income. 3. Operating cash flow excludes non-cash items. 4. % increase in cash reserves compared to previous quarter. |
||
The results reported for the quarter ended 30 June 2022 reflect the work of CLINUVEL’s team to enable wider reimbursement for SCENESSE® treatment, with more centres facilitating access for more patients. These activities drove the highest cash receipts recorded for a June quarter which were 64% higher than the June quarter of 2021.
June quarter cash expenditures were lower than the previous four quarters due to a temporary reduction in supply chain and manufacturing costs. CLINUVEL’s commitment to managing expenditures in support of growth is expected to #continue, consistent with the Company’s stated projections of A$175 million over the five years to 30 June 2025.
The net outcome of the higher cash receipts and lower cash expenditures was a strong rise in cash inflow in the quarter of A$18.478 million. This boosted cash reserves by 19.3% to A$121.509 million. The deployment of the accumulated cash reserves is discussed below.
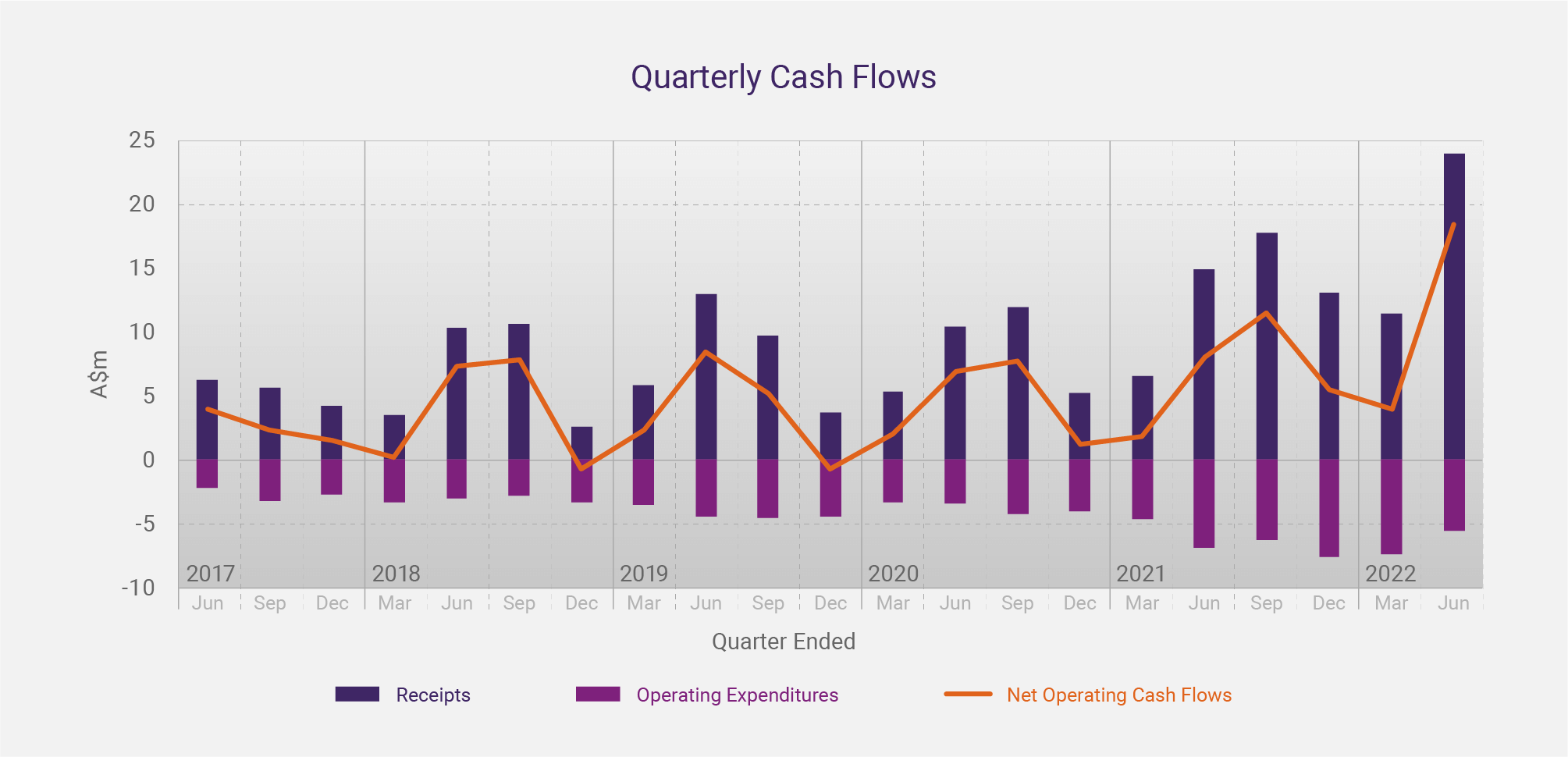
The graph below shows CLINUVEL’s quarterly cash flow data over the past five years. The increase in cash receipts and net cash inflow in recent years, concurrent with the distribution of SCENESSE® in the USA since April 2020, is evident.
CLINUVEL’s cashflows exhibit seasonality and strong annual growth
Seasonality has been less of a feature of CLINUVEL’s quarterly cash receipts due to the experience centres have gained with the prescribed drug. We now see that physicians are gradually prescribing more doses per year. Since each dose of SCENESSE® provides photoprotection for 60 days, many patients seem to benefit from all-year-round treatment.
Gradually, we see the effects of seasonality on cash receipts being ironed out. After growth of free cash in previous years, there was a decline of 9.1% in the year to 30 June 2020 when the initial impact of COVID-19 in Europe limited the capacity of some hospitals and the mobility of some patients to provide and receive treatment, respectively. The cash receipts normalised in successive quarters of FY2021 and growth of 32.2% occurred in the year to 30 June 2021, the first full year of commercial operations in the US.
The results reported for the quarter ended 30 June 2022 reflect the work of CLINUVEL’s team to enable wider reimbursement for SCENESSE® treatment, with more centres facilitating access for more patients.
Deployment of Accumulated Cash Reserves
(Lachlan Hay, Director of Global Operations)
CLINUVEL has maintained a financial control over the last decades, for deployment of capital to meet business objectives. The Board supports three uses of accumulated cash reserves as part of an overall capital management plan.
First, the principal use of the cash for financing the Company’s planned organic growth, including the clinical and regulatory programs. As mentioned above, CLINUVEL has announced a plan to expend A$175m over the five-years to June 2025 in support of expansion. We seem to be on track to achieve the projections, but the final year results will need to confirm.
Second, the cash at hand provides a buffer to manage adverse economic cycles and unexpected events. The value of the Company’s financial conservatism to build a significant cash reserve cannot be understated. The need of many peers to source cash from the markets has been exacerbated by the difficulties of the current operating environment, and loss of value in life sciences. Under current conditions, capital markets – understandably – have less appetite and financiers are more expensive and demanding on the terms and conditions of their finance. We believe that raising funds on a regular basis is not in the interest of shareholders. CLINUVEL shareholders have not been diluted since the Company last raised capital in March 2016, and the Company has navigated both the 2007/08 GFC and recent COVID-19 pandemic without needing to employ drastic measures to ensure access to capital.
Third, the cash reserves enable:
- capital expenditure on new assets; and
- the acquisition of assets and/or companies.
The value of the Company’s financial conservatism to build a significant cash reserve cannot be understated.
Communications and Investor Relations
(Mr Malcolm Bull, Head of Australian Operations and Investor Relations)
Recent announcements
Company announcements to the Australian Securities Exchange since the last news communiqué (on 23 June) are listed below:
| Date | Announcement |
|---|---|
| 25 Jul | Appendix C, Cash Receipts and Activities Report – June quarter 2022 |
| 28 Jul | PRÉNUMBRA® for Stroke |
| 01 Aug | Chair’s Letter II – 2022 |
| 04 Aug | News Communiqué IV – 2022 |
All CLINUVEL’s announcements are available on the CLINUVEL website and CLINUVELNews. More specifically, announcements to the Australian Securities Exchange are available on the investor pages of the CLINUVEL website.
Key announcements and events, rest of 2022
The Investor Relations calendar for the remainder of 2022 is filled with the following key announcements and events:
| Date | Announcement / Event |
|---|---|
| By end Aug | Appendix 4E and Annual Report – Financial Year Ended 30 June 2022 |
| Annual Results FY2022, Investor Briefing | |
| 10-12 Sep | H.C. Wainwright Annual Global Investment Conference, New York |
| 17 Sep | Shareholder Briefing, Monaco |
| Mid Oct | Shareholder Briefing, Sydney |
| Late Oct | Annual General Meeting of Shareholders |
| 15-17 Nov | Jefferies Healthcare Conference, London |
We expect to participate in other conferences, such as the Morgan Scone Value in the Vines Conference. The shareholder briefings planned for Monaco and Sydney in September and October, respectively continue the Company’s return to face-to-face interaction with fund managers, banks, new investors, journalists, and analysts, as advised in News Communiqué III – 2022. Details of the August Investor Briefing and the Annual General Meeting will be made available in due course. Shareholders interested to ask questions ahead of the August Investor Briefing may table them through the Investor Relations Contact Form available on the Company’s website.
Summary and conclusion
CLINUVEL’s mission started by focussing on melanocortins, human hormone analogues, and it evolved by translating its knowledge and scientific data to larger populations. While many other companies have focussed on this class of hormones, the challenges to turn this focus into economic success have remained. We believe that the key to delivering scientific concepts and breakthrough products lies in our personnel’s continued commitment. With much buoyancy I thank you for your show of support.
Malcolm Bull, Head of Australian Operations & Investor Relations
The shareholder briefings planned for Monaco and Sydney in September and October, respectively continue the Company’s return to face-to-face interaction with fund managers, banks, new investors, journalists, and analysts…
References
- Minder, et al (2021). Beyond pigmentation: Signs of liver protection during afamelanotide treatment in Swiss patients with erythropoietic protoporphyria, an observational study. Therapeutic Advances in Rare Disease. 2: 1-17.
- Wensink, et al (2020). Association of Afamelanotide With Improved Outcomes in Patients With Erythropoietic Protoporphyria in Clinical Practice. JAMA Dermatology. 156(5): 570–575.
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD.
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products; the COVID-19 pandemic and/or other world, regional or national events affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical products; competition for our products, especially SCENESSE® (afamelanotide 16mg), PRÉNUMBRA® or NEURACTHEL®; our ability to achieve expected safety and efficacy results in a timely manner through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, Israel, China and Japan of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE®, PRÉNUMBRA® or NEURACTHEL® which may lead to it being unable to supply its commercial markets and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology and consumer based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; our ability to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2021 Annual Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on preliminary and uncertain forecasts and estimates is available on request, whereby it is stated that past performance is not an indicator of future performance.
Contact
Level 11, 535 Bourke St
Melbourne, 3000 Vic,
Australia
+61 3 9660 4900
+61 3 9660 4909